
��ѧ��ӦN2+3H2=2NH3�������仯����13ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
| A��N2(g)+3H2(g)=2NH3(1); ��H=2(a-b-c)kJ��mol-1 |
| B��N2(g)+3H2(g)=2NH3(g); ��H=2(b-a)kJ��mol-1 |
C��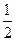 N2(g)+ N2(g)+ H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1 H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1 |
D��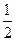 N2(g)+ N2(g)+ H2(g)=NH3(g); ��H=(a+b)kJ��mol-1 H2(g)=NH3(g); ��H=(a+b)kJ��mol-1 |
 С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?���죩��ѧ��ӦN2+3H2=2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������
��2008?���죩��ѧ��ӦN2+3H2=2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��ӦN2+3H2��2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������
��ѧ��ӦN2+3H2��2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������| A��N2��g��+H2��g����NH3��1��-46 kJ | B��N2��g��+H2��g����NH3��g��-454 kJ | C��N2��g��+3 H2��g����2 NH3��g��+92 kJ | D��N2��g��+3 H2��g����2 NH3��1��+431.3 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
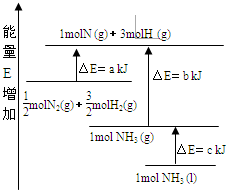 ��ѧ��ӦN2+3H2?2NH3�������仯��ͼ��ʾ��E����ֵ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������
��ѧ��ӦN2+3H2?2NH3�������仯��ͼ��ʾ��E����ֵ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������| A��N2��g��+3H2��g��?2NH3��l������H=2��a-b-c��kJ?mol-1 | ||||
| B��N2��g��+3H2��g��?2NH3��g������H=2��b-a��kJ?mol-1 | ||||
C��
| ||||
D��
|
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com