
����Ŀ���л���K�����Ƹ�Ѫѹҩ�����Ҫ�м��壬���ĺϳ�·������(����ת��������ȥ)
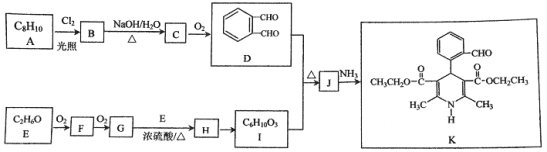
��֪��
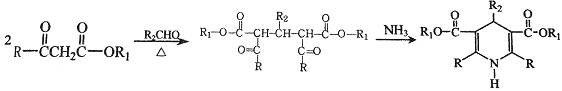
����
����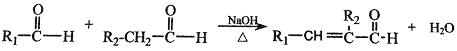 (R��R1��R2��ʾ��ԭ�ӻ�����)
(R��R1��R2��ʾ��ԭ�ӻ�����)
��1��A�Ľṹ��ʽ��__________��
��2��G��E����H�Ļ�ѧ����ʽ��__________.
��3��C��D�Ļ�ѧ����ʽ��__________.
��4��I�Ľṹ��ʽ��__________��
��5���й�J��˵���У���ȷ����__________(ѡ����ĸ)��
a������NaHCO3��Ӧb������NaOH��Ӧc����������Cu(OH)2��Ӧ
��6��K��������ת����ϵ��K![]() M(C16H15NO5Na2)��M�Ľṹ��ʽ��__________��
M(C16H15NO5Na2)��M�Ľṹ��ʽ��__________��
���𰸡���1��![]() ����2��CH3COOH+C2H5OH
����2��CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��3��![]() +O2
+O2 ![]()
![]() +2H2O��
+2H2O��
��4��![]() ����5��bc����6��
����5��bc����6�� ��
��
��������
������������A�ķ���ʽ��D�Ľṹ����֪AΪ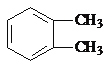 ����BΪ
����BΪ![]() ��CΪ
��CΪ![]() ��E������������������Ӧ�����E�ķ���ʽ��֪EΪC2H5OH����FΪCH3CHO��GΪCH3COOH��HΪCH3COOC2H5��I��C��Ӧ�õ�J��J�백����Ӧ�õ�K����K�Ľṹ���ƽ����Ϣ����֪JΪ
��E������������������Ӧ�����E�ķ���ʽ��֪EΪC2H5OH����FΪCH3CHO��GΪCH3COOH��HΪCH3COOC2H5��I��C��Ӧ�õ�J��J�백����Ӧ�õ�K����K�Ľṹ���ƽ����Ϣ����֪JΪ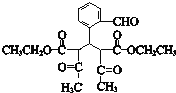 ��IΪ
��IΪ![]() ��
��
��1��A�Ľṹ��ʽ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��G��E����H�Ļ�ѧ����ʽ�ǣ�CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��3��C��D�Ļ�ѧ����ʽ�ǣ�![]() +O2
+O2 ![]()
![]() +2H2O���ʴ�Ϊ��
+2H2O���ʴ�Ϊ��![]() +O2
+O2 ![]()
![]() +2H2O��
+2H2O��
��4��I�Ľṹ��ʽ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5��JΪ ��a��û���Ȼ���������NaHCO3��Ӧ����a����b����������������NaOH��Ӧ����b��ȷ��c������ȩ������������Cu(OH)2��Ӧ����c��ȷ����ѡ��bc��
��a��û���Ȼ���������NaHCO3��Ӧ����a����b����������������NaOH��Ӧ����b��ȷ��c������ȩ������������Cu(OH)2��Ӧ����c��ȷ����ѡ��bc��
��6��K��������ת����ϵ��K![]() M(C16H15NO5Na2)��������Ϣ���з�Ӧ��������������ˮ�ⷴӦ����M�Ľṹ��ʽ��
M(C16H15NO5Na2)��������Ϣ���з�Ӧ��������������ˮ�ⷴӦ����M�Ľṹ��ʽ��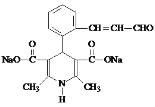 ���ʴ�Ϊ��
���ʴ�Ϊ��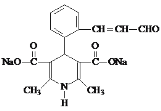 ��
��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����N��B��Ԫ����ɵ����Ͳ������Ź㷺��;��
��1��B2H6��һ�ָ���ȼ�ϣ�����Cl2��Ӧ���ɵ�BCl3�����ڰ뵼����ӹ��ռ��ߴ������죬�ɵڶ�����Ԫ����ɵ���BCl3��Ϊ�ȵ������������Ϊ_________(�����ӷ��ţ���һ��)��
��2�������黯����(H2N��BH2)��Ti(BH4)3��Ϊ���ܹ�ע�����ͻ�ѧ�����ﴢ����ϡ�
��H2N��BH2��Nԭ�ӵ��ӻ�����Ϊ_________��
��Ti(BH4)3��TiCl3��LiBH4��Ӧ�Ƶá���̬Ti3+��δ�ɶԵ�������____����BH4-�����幹����_________��д�����Ʊ���Ӧ�Ļ�ѧ����ʽ_________��
�������������Ԫ��״������(HB=NH)3ͨ����������Ӧ�Ƶã�
3CH4+2(HB=NH)3+6H2O�T3CO2+6H3BNH3��������ѧ����ʽ�йص���������ȷ����_________����(����)
A���������д�����λ��
B����һ�����ܣ�N��O��C��B
C����Ӧǰ��̼ԭ�ӵĹ���ӻ����Ͳ���
D��CH4��H2O��CO2���ӿռ乹�ͷֱ��ǣ����������Ρ�V�Ρ�ֱ����
��3������(BP)���ܵ��߶ȹ�ע�����O���ϣ���ͼ1Ϊ������
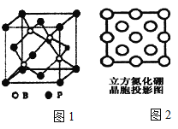
������������________����(�������)��________(���ǻ��)������λ����
��������Bԭ�ӵ���λ��Ϊ_______��
��4��������������һ�����͵ij�Ӳ�����O�����µĽṹ���ϣ���ṹ��Ӳ�ȶ�����ʯ���ƣ����۵�Ƚ��ʯ�ͣ�ԭ����________��ͼ2��������������z���ͶӰͼ������ͼ��Բ����Ϳ���ͻ��������ֱ����B��N�����λ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ����ѡ��5���л���ѧ����]
�ҹ�����ҩѧ����������������ű������ҩ�������غ�˫�������ض���2015��ŵ��������ѧ��ҽѧ���������磬�ж��й�����֪��������һ�ֻ�ѧ���ֹ����������£�
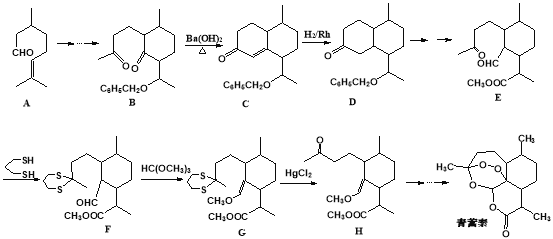
��֪�� 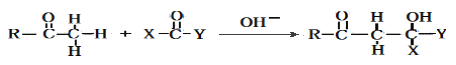
��1�������й�˵������ȷ����______________
A����������ʪ��ĵ��۵⻯����ֽ��������ɫ������Ϊ���ӽṹ�к�������
B��������������ˮ���������Ҵ�������
C�����������ڻ�״������������ڷ����廯����
D��һ�������£���������������������Һ��Ӧ
��2��������A�к��еķǺ��������ŵ�������________________________����ѡ�����к��ʵ��Լ�������ù����ţ��Լ��������ȷ˳��Ϊ_______________��
A����ˮ B��ϡ���� C������������ͭ����Һ D������������Һ
��3���ù������������E��F��G��H��Ŀ����________________________ ��
��4����ӦB��C��ʵ���Ͽɿ����������С��Ը�����֪��Ϣд�����η�����Ӧ�ķ�Ӧ������_______________��________________��
��5��M��A��Ϊͬϵ�����A������̼ԭ�ӡ���������������M��ͬ���칹����___��(�����������칹)���� ������Ԫ�� ���ܷ���������Ӧ
��6��������ѧ֪ʶ��������Ϣ��д���ɱ���ȩ��������Ϊԭ�ϣ����Լ����ã����Ʊ��л���ȩ��![]() ����·������ͼ��·������ͼʾ�����£�
����·������ͼ��·������ͼʾ�����£�
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���A����ͨ����ͬ�ķ�Ӧ�õ�B��C:
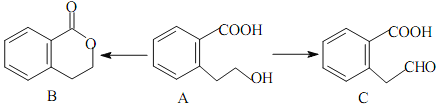
��1��A�ķ���ʽΪ_________________��C�ĺ�������������Ϊ_________________��
��2��A��ȡC���л���Ӧ����Ϊ_________________��A��ȡB�Ļ�ѧ����ʽΪ��_________________��
��3��A������ȥ��Ӧ�����Ľṹ��ʽΪ_________________��A������һ�������̼ԭ����_________________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ɱ�ϩ�����з�Ӧ���Ƶ�F��G���ָ߷��ӻ�������Ƕ��dz��õ����ϡ���������E���緢������ţ���У������������Ǵ�л���м��壬�����������������۵ȷ����Ƶã�E�ĸ���������ϲ���IJ��Ƽ�֮һ��
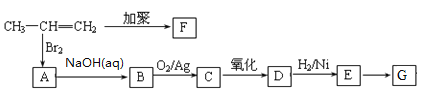
��֪��![]()
��1��D���������������ơ�E��G�ķ�Ӧ����Ϊ_________��
��2���ۺ���F�Ľṹ��ʽ���ۺ���G�Ľṹ��ʽ_________
��3����һ�������£�������E��Ũ�����������γ�һ����Ԫ��״������û�����Ľṹ��ʽ��_________��
��4��Bת��ΪC�Ļ�ѧ��Ӧ����ʽ��_________��
��5���������ֻ�������E��Ϊͬ���칹�����_________��
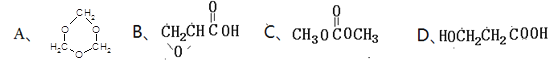
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС����̼�ظ�(����CԪ��0.03%~2.0%���Ͻ�)����������̽�����
[̽��һ]
��1������ȥ�����������������������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ����____��
��2��ȡ̼�ظ�6.0 g��15.0 mLŨ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ����������Y��
�ټ�ͬѧ��ΪX�г�Fe3���������Fe2������Ҫȷ�����е�Fe2����Ӧѡ��____��
A��KSCN��Һ����ˮ B�����ۺ�KSCN��ҺC��Ũ��ˮD������KMnO4��Һ
����ͬѧȡ560 mL(��״��)����Yͨ��������ˮ�У�����SO2��Br2��2H2O===2HBr��H2SO4��Ӧ��Ȼ���������BaCl2��Һ�����ʵ�������ø������4.66 g���ɴ���֪����Y��SO2���������Ϊ________��
[̽����]��������ʵ����SO2��������ķ�������ͬѧ��Ϊ����Y�л����ܺ���Q1��Q2�������壬����Q1���壬�ڱ�״���£��ܶ�Ϊ0.089 3 g��L��1��Ϊ�����������̽��ʵ��װ��(�����й�������ȫ��Ӧ)��
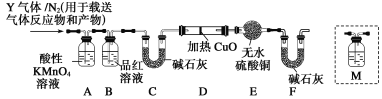
��3��װ��B���Լ���������________________________��
��4������Y�����е�Q2������������ɵ�______________(�û�ѧ����ʽ��ʾ)��
��5����֪ϴ��ƿM��ʢװ����ʯ��ˮ��Ϊȷ��Q2�Ĵ��ڣ�����װ��������ϴ��ƿM��________(�����)��
A��A֮ǰ B��A��B�� C��B��C�� D��C��D��
��6���������Y�к���Q1��Ԥ��ʵ������Ӧ��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����к˵����С��20��Ԫ��A����������������£�(I1��ʾʧȥ��1�����ӵĵ����ܣ�In��ʾԭ��ʧȥ��n�����ӵĵ����ܣ���λ����102kJ��mol��1)
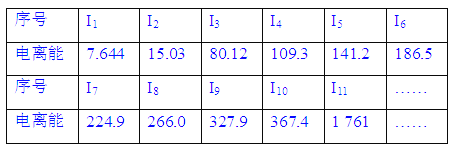
��1�����������ԽԶ������Խ�ߣ�������Խ__________(������������С��)�������ӵ����Խ�ߣ�ʧȥ����ʱ��������Խ________(������������С��)��
��2������11�����ӷ���________�����Ӳ㡣
��3��ʧȥ��11�����Ӻ�Ԫ�ػ���________�����ӡ�
��4����Ԫ������������Ӧˮ����Ļ�ѧʽ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ��ѡ��3�� ���ʽṹ�����ʡ��ѡ�������������ͭ�Ƚ������仯�����ڹ�ҵ������Ҫ��;��
��1�������Ͻ�����ϵ����Ͻ�Ĵ������úϽ���з����¶ȵ͡��۸����е��ŵ㡣
��Ti�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ________________��
��Fe�Ļ�̬ԭ�ӹ���________�ֲ�ͬ�ܼ��ĵ��ӡ�
��2���Ʊ�CrO2Cl2�ķ�ӦΪK2Cr2O7��3CCl4===2KCl��2CrO2Cl2��3COCl2����
��������ѧ����ʽ�зǽ���Ԫ�ص縺���ɴ�С��˳����______________(��Ԫ�ط��ű�ʾ)��
��COCl2����������ԭ�Ӿ�����8���ӹ��ͣ�COCl2�����ЦҼ��ͦм��ĸ�����Ϊ________������ԭ�ӵ��ӻ���ʽΪ________��
��3��NiO��FeO�ľ���ṹ�����Ȼ��Ƶľ���ṹ��ͬ������Ni2����Fe2�������Ӱ뾶�ֱ�Ϊ6.9��10��2 nm��7.8��10��2 nm�����۵㣺NiO________(���������<������)FeO��
��4��Ni��La�ĺϽ���Ŀǰʹ�ù㷺�Ĵ�����ϣ����д����������������͵��µ��ص㣬���ձ����й���ʵ���˲�ҵ����
�úϽ�ľ����ṹ��ͼ��ʾ��

���þ���Ļ�ѧʽΪ________________��
����֪�þ�����Ħ������ΪM g��mol��1���ܶ�Ϊd g��cm��3����NAΪ�����ӵ�������ֵ����þ����������________ cm3(�ú�M��d��NA�Ĵ���ʽ��ʾ)��
���þ�����ڲ����п�϶����ÿ�������Ŀ�϶�д���6����ԭ�ӱȽ��ȶ�����֪��a��511 pm��c��397 pm����״�����������ܶ�Ϊ8.98��10��5 g��cm��3������������![]() ������������ǰ��������仯����ô�����ϵĴ�������Ϊ_______��
������������ǰ��������仯����ô�����ϵĴ�������Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ������
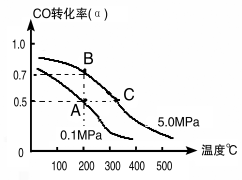
��1�����ȡ��CO��H2Ϊԭ�Ϻϳ���������ѧ��Ӧ����ʽ��2CO(g)+4H2(g)![]() CH3CH2OH(g)+H2O(g) ��H�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3CH2OH(g)+H2O(g) ��H�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��֪��2CO(g)+O2(g)===2CO2(g) ��H1=��566kJ��mol��1
2H2(g)+O2(g)===2H2O(l) ��H2=��572kJ��mol��1
CH3CH2OH(g)+3O2(g)===2CO2(g)+ 3H2O(g) ��H3=��1366kJ��mol��1
H2O(g)===H2O(l) ��H4=��44kJ��mol��1
����H= kJ��mol��1
����A��C���㶼��ʾ�ﵽ��ƽ��״̬�������Ӧ��ʼ����ƽ��״̬�����ʱ��tA tC�������������������������
����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱ��A��ʱ���������Ϊ10L������¶��µ�ƽ�ⳣ����K�� ��
������̼����ȼ�ϵ�أ�MCFS��������ú����CO+H2��������ȼ����������CO2�Ļ����Ϊ������ȼ������һ������Li2CO3��Na2CO3�����������������ʣ��Խ�������ȼ�ϼ���Ϊ�����Ƴɵġ�������CO��Ӧ�ĵ缫��ӦʽΪ ��
��2����ҵ�ϻ����Բ�ȡ��CO2��H2Ϊԭ�Ϻϳ��Ҵ������Ҹ�����ѧ�������Ƴ磬��������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH ��ƽ�ⳣ�������Ʋ⻯ѧ�������Ͽ���CO2��ȡCH3CH2OH���ŵ���Ҫ�ǣ� ��
��3��Ŀǰ��ҵ��Ҳ������CO2�������״���һ�������·�����ӦCO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���
CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���

�����ڴ����ͼ�л���״������ʵ�����ʱ��仯���ߡ�
�ڽ��ı�ijһʵ�������ٽ�������ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı��� �����ߢ��Ӧ��ʵ�������ı��� ��
��4������״����4.48L CO2ͨ��1L 0.3mol��L��1 NaOH��Һ����ȫ��Ӧ��������Һ����Ũ�ȹ�ϵ��ȷ����
A��c(Na+)=c(HCO3-)��c(CO32-)��c(H2CO3)
B��c(OH-)+c(CO32-)=c(H2CO3)��c(H+)
C��c(Na+)��c(H+)=c(HCO3-)��2c(CO32-)��c(OH-)
D��2c(Na+)=3c(HCO3-)��3c(CO32-)��3c(H2CO3)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com