
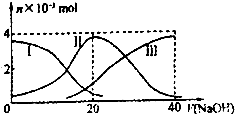
 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ��� �仯��ͼ������I����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ��� �仯��ͼ������I����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������| A����V��NaOH��=20mLʱ����Һ������Ũ�ȴ�С��ϵ c��Na+����c��HA-����c��H+����c��A2-����c��OH-�� |
| B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� |
| C����ʹNaHA��Һ�����ԣ����������м������� |
| D����NaHA��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ǽ���Ԫ�أ�ϡ������Ԫ�س��⣩��������Ԫ�أ������ڷ�Ӧ�ж�ֻ���������� |
| B�������£�1mol����������ϡNaOH��Һ��ȫ��Ӧת��1mol���� |
| C���ڼ��������£����Ҵ���ȥ���������е����� |
| D������ͭ��Һ�����Ե�ԭ��Cu2++2H2O�TCu��OH��2��+2H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ��16Oԭ�ӵ�����Ϊ16 g | ||
| B����Ԫ�صĽ������ԭ������Ϊ��16a%+17b%+18c%�� | ||
C����Ԫ�صĽ������ԭ������Ϊ��
| ||
| D����Ԫ�ص����ԭ������Ϊ��16a%+17b%+18c%�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����λʱ��������n mol A2��ͬʱ����n mol B2 |
| B�������ڣ�AB��A2��B2�������干�� |
| C��AB���������ʵ���A2���������� |
| D�������и���ֵ������������ʱ��仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1��2 | B��2��1 |
| C��3��2 | D��2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ֵ�Ũ�Ȳ��ٱ仯 |
| B�����淴Ӧ���ʾ�Ϊ�� |
| C����Ӧ��Ũ��С���������Ũ�� |
| D����Ӧֹͣ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | �������� | ��ȥ���ʵķ��� | |
| A | CO | CO2 | ͨ����������������Һ������ |
| B | NaCl | ��ɳ | �ܽ⡢���ˡ����� |
| C | NaOH��Һ | Na2CO3 | ��������ϡ���������ٲ������� |
| D | Cu��NO3��2��Һ | AgNO3 | ���������ͭ�ۣ����� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��N2O4��g��?2NO2��g����H=+57.20kJ?mol-1��
��֪��N2O4��g��?2NO2��g����H=+57.20kJ?mol-1��| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| c��N2O4��/mol/L | 0.100 | c1 | 0.050 | c3 | c4 |
| c��NO2��/mol/L | 0.000 | 0.060 | c2 | 0.120 | 0.120 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��HCO3-����c��CO32-�� |
| B��c��HCO3-����c��HSO3-�� |
| C��c��CO32-��+c��HCO3-���Tc��SO32-��+c��HSO3-�� |
| D������Һ����ʹ���Ը��������Һ��ɫ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com