
������ѧϰ���о���ѧ��һ�ֳ��õĿ�ѧ���������з����������
�ٸ���������к��е�Hԭ�Ӹ��������ΪһԪ�ᡢ��Ԫ�ᡢ��Ԫ��
�ڸ��ݷ�Ӧ���Ƿ��е���ת�ƽ���ѧ��Ӧ��Ϊ������ԭ��Ӧ�ͷ�������ԭ��Ӧ
��ͬλ�أ�1H��2H��3H���ɱ���Һ�ȶ��Ƿǵ����
�ܵ���ʣ������������ᡢ���������ͬ�������壺C60�����ʯ��ʯī
�ݸ��ݷ�ɢϵ���ȶ��Դ�С��������Ϊ���塢��Һ����Һ
| A��ֻ�Тڢ� | B��ֻ�Тڢۢ� | C��ֻ�Т٢ڢ� | D��ֻ�Тڢۢ� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й������ʷ������ȷ����ǣ� ��
| | �� | �� | �� | ���������� | ���������� |
| A | ���� | ���� | С�մ� | ������ | �ɱ� |
| B | ������ | ���� | ʳ�� | ������ | һ����̼ |
| C | ��ʯ�� | ������(CH3COOH) | ����CuSO4��5H2O | �������� | �������� |
| D | ���Լ� | HNO3 | ̼��� | ��ʯ�� | SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ͨ��������Һʱ���Ӳ���۲쵽һ�������ġ�ͨ·����˵��������Һ��
| A������ | B������Һ | C����Һ | D������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵����ȷ����
A����֪KCl��MgO�ľ���ṹ��NaCl�ľ���ṹ���ƣ�������۵㣺MgO>KCl>NaCl
B ����з�̪��̼������Һ�м���BaCl2��Һ����Һ��ɫ
C����ɢϵ�з�ɢ�����ӵ�ֱ����Fe(OH)3����>Fe(OH)3����Һ>FeCl3��Һ
D��Na2O��MgO��Al2O3�����ڼ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и������ʵķ�����ȷ����
| A��ͬλ�أ�1H+��2H2��3H |
| B��ͬϵ����顢�춡�顢������ |
| C������ʣ� �����ᡢˮ�����ռ� |
| D�����������һ�������p������̼���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ڻ�ѧѧ�Ƶķ�չ�����˷dz���Ҫ�����á�ͼ1��ij��Ӧ���ܱ������з�Ӧǰ��ķ���״��ʾ��ͼ���� ���͡�
���͡� ���ֱ��ʾ��ͬ��ԭ�ӡ��Դ˷�Ӧ�ķ���һ������ȷ����
���ֱ��ʾ��ͬ��ԭ�ӡ��Դ˷�Ӧ�ķ���һ������ȷ����
| A���û���Ӧ | B��������ԭ��Ӧ | C�����ȷ�Ӧ | D�����Ϸ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��17�֣��������������ʣ��پƾ�����ͭ���������������ܰ����������ǡ� �����ᡢ��̼�����ơ������ᡢ�����⡢��Al2(SO4)3���������ʵ������д���пհ�
��1������ǿ����ʵ��У� ��
��2��Һ̬ʱ�ܵ�����Ϊ�����仯���У� ��
��3������ˮ��Һ�ĵ��뷽��ʽΪ ��
��A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��
��1����A�ǵ���ɫ���壬C��D���������C������������Ҫ���ʡ�����C���ʿɵõ��м�ֵ�Ļ�ѧƷ��д���û�ѧƷ�е�1�����1���ε������� ������������ ��������
��2����B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�����Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ������������ �� ������ ����������
��3����D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���塣�жϵ���A��Ԫ�������ڱ��е�λ����___ _____��д�ܷ�Ӧ���ӷ�����������
��4����A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڢݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬д�÷�Ӧ�����ӷ���ʽ����������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
��1��NH4Al(SO4)2������ˮ������������ (�ñ�Ҫ�Ļ�ѧ������������˵��)��
��2����ͬ�����£�0��1 mol��L��1NH4Al(SO4)2��c(NH4+) (����ڡ��������ڡ���С�ڡ�)0��1 mol��L��1NH4HSO4��c(NH4+)��
��3��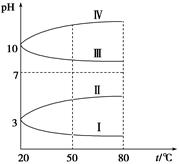
��ͼ��0��1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0��1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯�������� (��д��ĸ)������pH���¶ȱ仯��ԭ���� ��
��20 ��ʱ��0��1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)�� ��
��4������ʱ����100 mL 0��1 mol��L��1NH4HSO4��Һ�еμ�0��1 mol��L��1NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������� ����b�㣬��Һ�и�����Ũ���ɴ�С������˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���и������ʣ��� O2��O3�� �� 12C��14C���� CH3CH2CH2CH3��(CH3)2CHCH3��������Ͷ��飻�� CH3CH2CH2CH(C2H5)CH3�� CH3CH2CH2CH(CH3)C2H5��
��Ϊͬϵ����� ����Ϊͬ���칹����� ����Ϊͬλ�ص��� ����Ϊͬ����������� ����ͬһ���ʵ��� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com