������ʵĵ���ƽ�⡢�����ˮ��ƽ������������Һƽ������ڶ�̬ƽ�⣮
��1����֪H
2A��ˮ�д�������ƽ�⣺H
2A=H
++HA
-��HA
-?H
++A
2-��
��NaHA��Һ
������
������
��ѡ������ԡ������Լ��ԡ����������ԡ�����ȷ��������
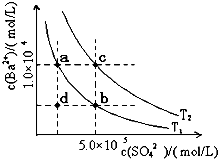
����֪������H
2A�ĸ��Σ�CaA��������Һ�д�������ƽ�⣺CaA��s��?Ca
2+��aq��+A
2-��aq����H��0�������¶�ʱ��K
sp��С
��С
�����������С�����䡱����ͬ�����μ�����Ũ���ᣬc��Ca
2+��
����
����
��
��2����֪ˮ�ĵ��뷽��ʽ��дΪ2H
2O?H
3O
++OH
-��Һ��������ˮ�ĵ��룬��Һ���ĵ��뷽��ʽΪ
2NH3?NH2-+NH4+
2NH3?NH2-+NH4+
����Һ���м���NH
4Cl����ƽ�⽫��
����
����
�ƶ��������������
��3�������£���ijpH=11��Na
2CO
3��Һ�м������ʯ���飬���˺�������ҺpH=13����Ӧǰ����Һ���뷴Ӧ�����Һ��ˮ�������c��OH
-���ı�ֵ��
1��1010
1��1010
��
��4����������ˮ�õ���ˮ����25���£���a mol?L
-1�İ�ˮ��b mol?L
-1������������ϣ���Ӧ����Һ�����ԣ����ú�a��b�Ĵ���ʽ��ʾ���û����Һ�а�ˮ�ĵ���ƽ�ⳣ��
��



 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ���������±���
��֪25��ʱ����������ʵĵ���ƽ�ⳣ���������±���