
| A������Һ�п��ܺ�K+ |
| B������Һ�п϶�����NO3?��SO42-��NH4+��CO32- |
| C������Һ��һ������NH4+ |
| D������Һ��һ����K+����c��K+����0.1mol/L |
| 0.224L |
| 22.4L/mol |
| 2.33g |
| 233g/mol |
| 0.01mol��2-0.01mol |
| 0.1L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ԫ�� | B����Ԫ�� |
| C��ά���� | D����Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
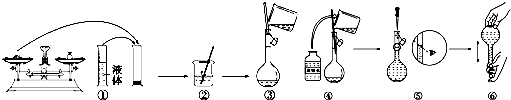
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� |
B�� |
C�� |
D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | |||
| B | C | D |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
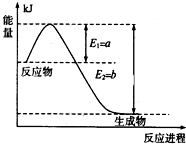 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� 2��3���� |
B�� 3�һ�1��ϩ |
| C����CH3CH2��2CHCH3��3������ |
D�� ������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com