
������(H3PO2)��һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԡ��ش��������⣺
(1)H3PO2��һ����ǿ�ᣬд������뷽��ʽ��_____________________________
________________________________________________________________________��
(2)H3PO2��NaH2PO2���ɽ���Һ�е�Ag����ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ________��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ________(�ѧʽ)��
��NaH2PO2Ϊ________(����Ρ�����ʽ�Ρ�)������Һ��________(������ԡ������ԡ��������ԡ�)��
(3)H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(4)H3PO2Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)��

��д�������ĵ缫��Ӧʽ��_________________________________________��
�ڷ�����Ʒ�ҿɵõ�H3PO2��ԭ��_____________________________________
________________________________________________________________________��
�����ڲ��á����ҵ����������Ʊ�H3PO2���������ҵ����������������ҵ�ϡ������H3PO2ϡ��Һ���棬����ȥ���������Ʒ��֮�����Ĥ���Ӷ��ϲ������������Ʒ�ҡ���ȱ���Dz�Ʒ�л���________���ʣ������ʲ�����ԭ����________________________________��
(1)H3PO2H2PO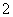 ��H����(2)�٣�1����H3PO4�������Ρ�������
��H����(2)�٣�1����H3PO4�������Ρ�������
(3)2P4��3Ba(OH)2��6H2O��3Ba(H2PO2)2��2PH3��
(4)��2H2O��4e��===O2����4H��
�������ҵ�H��������Ĥ��ɢ����Ʒ�ң�ԭ���ҵ�H2PO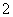 ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO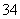 ��H2PO
��H2PO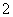 ��H3PO2������
��H3PO2������
[����] (1)H3PO2ΪһԪ���ᣬ����뷽��ʽΪH3PO2 H����H2PO
H����H2PO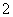 ��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO
��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO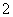 ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO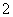 ��H3PO2ʧ���ӷ���������Ӧ�������������л���PO
��H3PO2ʧ���ӷ���������Ӧ�������������л���PO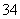 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ���������ʵ�����̣���һ��С�ձ������20g Ba(OH)2��8H2O�����10gNH4Cl���壬Ȼ��С�ձ����������ѵ���3�Ρ�4��ˮ�IJ���Ƭ�ϣ��������ò�����Ѹ�ٽ��衣ʵ������ʾ��ͼ���£��ش��������⣺

�� ʵ���в������������� ��
�� ����ϡ��������������� ��
�� ͨ�� ����˵���÷�ӦΪ ������ȡ����ȡ�����Ӧ���������ڷ�Ӧ��������� ���С�ڡ��� �����ڡ� ���ڡ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ�Ϊ1.00 L�������У�ͨ��һ������N2O4��������ӦN2O4(g)2NO2(g)�����¶����ߣ�����������ɫ���
�ش��������⣺
(1)��Ӧ�Ħ�H________0(����ڡ���С�ڡ�)��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________mol��L��1��s��1����Ӧ��ƽ�ⳣ��K1Ϊ________��
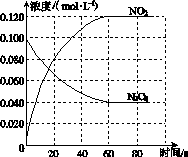
(2)100 ��ʱ��ƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.002 0 mol��L��1��s��1��ƽ�����ʽ��ͣ���10 s�ִﵽƽ�⡣
��T________100 ��(����ڡ���С�ڡ�)���ж�������____________________________��
����ʽ�����¶�Tʱ��Ӧ��ƽ�ⳣ��K2��_______________________________________
________________________________________________________________________��
(3)�¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����ж�������__________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̵�ص����С�������������dz��õ�һ�ε�ء��õ�ط�Ӧԭ����ͼ��ʾ�����е����LiClO4�����ڻ���л��ܼ��У�Li��ͨ�������Ǩ����MnO2�����У�����LiMnO2�� �ش��������⣺

(1)���·�ĵ�����������________������________����(����ĸ)
(2)���������ӦʽΪ__________________________��
(3)�Ƿ����ˮ�������еĻ���л��ܼ���________(��ǡ���)��ԭ����________________________________________________��
(4)MnO2����KOH��KClO3�ڸ����·�Ӧ������K2MnO4����Ӧ�Ļ�ѧ����ʽΪ____________________________________��K2MnO4��������Һ���绯������KMnO2��MnO2�����ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣ij�о�С���������̿�(��Ҫ�ɷ�ΪMnO2��������������������ͭ�����Ƚ���������)���������ͨ�����¼����̼��ѳ�ȼúβ���е�SO2�����Ƶõ�ز���MnO2(��Ӧ������ʡ��)��
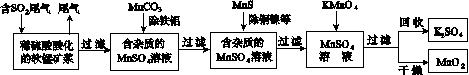
��ش��������⣺
(1)������������ʵ����____(ѡ��������ĸ���)��
A����������ۺ�����
B����ɫ��Ⱦ�ļ���
C������ļ���
(2)��MnCO3�ܳ�ȥ��Һ�е�Al3����Fe3������ԭ����________________________________��
(3)��֪��25 �桢101 kPaʱ��
Mn(s)��O2(g)===MnO2(s)����H����520 kJ/mol
S(s)��O2(g)===SO2(g)����H����297 kJ/mol
Mn(s)��S(s)��2O2(g)===MnSO4(s)��
��H����1065 kJ/mol
SO2��MnO2��Ӧ������ˮMnSO4���Ȼ�����ʽ��____________________________________��
(4)MnO2�����������������ϡ��ö��Ե缫���MnSO4��Һ���Ƶ�MnO2���������ĵ缫��Ӧʽ��________________________________��
(5)MnO2�Ǽ���п�̵�ص��������ϡ�����п�̵�طŵ�ʱ�������ĵ缫��Ӧʽ��________________��
(6)�����ѳ���SO2ֻ�����̿��е�MnO2��Ӧ������ͼʾ���̣���a m3(��״��)��SO2���������Ϊb%��β��ͨ�����SO2���ѳ���Ϊ89.6%�����յõ�MnO2������Ϊc kg�����ȥ��������ͭ����������ʱ�����������Ԫ���൱��MnO2________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ�������(����)
A����Ba(OH)2��Һ�еμ�ϡ���
Ba2����2OH����2H����SO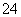 ===BaSO4����2H2O
===BaSO4����2H2O
B�����Խ�����KMnO4����H2O2��
2MnO ��5H2O2��6H��===2Mn2����5O2����8H2O
��5H2O2��6H��===2Mn2����5O2����8H2O
C�������ʵ�����MgCl2��Ba(OH)2��HCl��Һ��ϣ�Mg2����2OH��===Mg(OH)2��
D��Ǧ�����س��ʱ��������Ӧ��PbSO4��2H2O��2e��===PbO2��4H����SO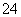
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯīΪ�缫�����KI��Һ(���к���������̪�͵���)������˵���������(����)
A������������Һ�ʺ�ɫ
B�������ݳ�����
C������������Һ����ɫ
D����Һ��pH��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������һ�����ᣬ��������ʴ��������֪25��ʱ
��HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H����67.7KJ��mol��1
��H+(aq)+OH��(aq)��H2O(l) ��H����57.3KJ��mol��1
��20mL0.1��molL��1������м���VmL0.1mol��L��1NaOH��Һ�������й�˵����ȷ����
A�������ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��
HF(aq)��H+(aq) +F−(aq) ��H��+10.4KJ��mol��1
B����V��20ʱ����Һ�У�c(OH��)��c(HF) +c(H+)
C����V��20ʱ����Һ�У�c(F��)��c(Na+)��0.1mol��L��1
D����V ��0ʱ����Һ��һ�����ڣ�c(Na+)��c(F��)��c(OH��)��c(H+)
��0ʱ����Һ��һ�����ڣ�c(Na+)��c(F��)��c(OH��)��c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���۽�����̼���κ���ʽ̼���εĻ�����������ϡ���ᷴӦ������H+������CO2���ʵ���֮��Ϊ6��5����û������̼���κ���ʽ̼���ε����ʵ���֮���� �� ��
A��1:1 B��1:2 C��1: 3 D��1:4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com