
��ҵ�����÷���м�����������������������ȣ�������ʽ������[Fe��OH��SO4]�Ĺ����������£�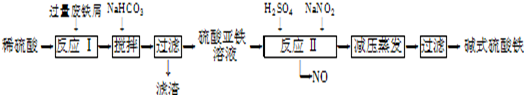
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe��OH��3 | Fe��OH��2 | Al��OH��3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������(��Ҫ�ɷ�ΪAl2O3��������������)����ȡ��������ԭ�ϡ���ȡ�������Ĺ����������£�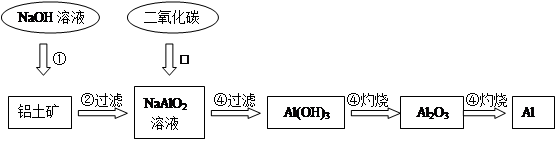
��1���������ӷ���ʽ��ʾ���Ϲ��������еڢٲ���Ӧ��_______ _______��
��2��д�����Ϲ��������еڢ۲���Ӧ�Ļ�ѧ����ʽ��______ ___________��
��3��������������������ڸ����£��ᷢ�����ҵķ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽ_____________�����һ���÷�Ӧ����;________________��
��4�����������������ȡ������������0.9mol���ӷ���ת�ƣ��������ܵõ���������������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��������Ʒ�к���������FeSO4���ʡ�ijͬѧҪ�ⶨ������Ԫ�ص���������������������·������вⶨ����������Ϊ��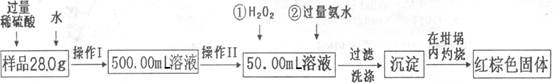
��������̻ش�
��1������I��������Һʱ�����õ��IJ����������ձ�����Ͳ������������ͷ�ι����⣬�������� (���������ƣ���
��2������II�б����õ��������� ��
| A��50mL��Ͳ | B��100mL��Ͳ |
| C��50mL��ʽ�ζ��� | D��50mL��ʽ�ζ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣���������ͭ���輰��Ͻ�������������������Ź㷺��Ӧ�á���ش������й����⡣
��1��Ŀǰ��ұ�������ȴ�99��9999�����������й��ڴ����������������
������ĸ����
| A��Ӳ�ȱȸ�С���۵�ȸָ� | B�����������ᷴӦ |
| C���벻��ֳɷ���ͬ | D�������Ũ�����жۻ� |
 6Cu+SO2�����÷�Ӧ��������������������
6Cu+SO2�����÷�Ӧ�������������������� 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��֪A��G����ͼ��ʾ��ת����ϵ����������������ȥ��������A��GΪ���ʣ�D����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬E��F������NaOH��Һ��Ӧ��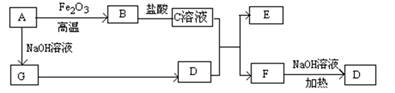
��ش��������⣺
��1��д��F�ĵ���ʽ ��
��2����C��Һ��D��Ӧ�����ӷ���ʽΪ ��
��F��Һ��NaOH��???���ȷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3�����������ӷ���ʽ����C��ҺΪ�������� ��
��F��Һ������Ũ���ɴ�С��˳��Ϊ ��
��4����5.4gAͶ��200mL 2.0mol/Lij��Һ����G���ʲ������ҳ�ַ�Ӧ���н���ʣ�࣬�����Һ������ ������ţ�
A��HNO3��Һ B��H2SO4��Һ C��NaOH��Һ D��HCl��Һ
��5����1molN2��3molG�����������ݻ�Ϊ2L��ij�ܱ������н��з�Ӧ����֪�÷�ӦΪ���ȷ�Ӧ��ƽ��ʱ�����D�����ʵ���Ũ��Ϊa mol/L��
�������Ӧ����v(G)��1.2mol/(L��min)����v(D)�� mol/(L��min)
���������������������£�����ʼʱ����0.5molN2��1.5molG�ﵽƽ���D�����ʵ���Ũ�� ������ڡ�����С�ڡ����ڡ���a/2 mol/L��
�۸������µ�ƽ�ⳣ��Ϊ ���ú�a�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��п��ͭ���ɵĺϽ�10 g������������ϡ�����г�ַ�Ӧ��������״��������2��24L������Ͻ���ͭ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ܵ����ʵ���Ϊ0.50 mol��Fe����Al�ۻ�����Ϊ�����������ݣ���һ���м�������
��ϡ���ᣬ�ڱ�״���²�������a L������һ���м�������������������Һ���ڱ�״���²�������b L����a+b����ֵ��������
| A��5.6 | B��7.3 | C��8.2 | D��11.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС��ͨ��ʵ���о�Na2O2��ˮ�ķ�Ӧ?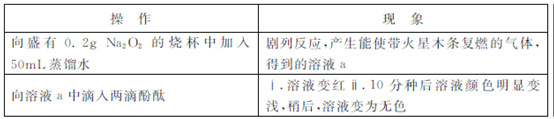
��1��Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ�� ?
��2��������Һ��ɫ��������Һa�д��ڽ϶��H2O2,H2O2���̪�����˷�Ӧ?
��ͬѧͨ��ʵ��֤ʵ��H2O2�Ĵ���:ȡ������Һa,�����Լ� (�ѧʽ)�����������?
����ͬѧ�������ϻ�Ϥ:��KMnO4(����ԭΪ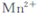 )���ԲⶨH2O2�ĺ���?
)���ԲⶨH2O2�ĺ���?
ȡ3mL��Һaϡ����15mL,��ϡH2SO4�ữ,����μ���0��0045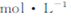 KMnO4��Һ,��������,��Һ��ɫ���ʿ�ʼ��������,���յ�ʱ������10mL KMnO4��Һ?
KMnO4��Һ,��������,��Һ��ɫ���ʿ�ʼ��������,���յ�ʱ������10mL KMnO4��Һ?
��KMnO4��H2O2��Ӧ�����ӷ���ʽ�� ?
����Һa�� ?
?
����Һ��ɫ���ʿ�ʼ���������ԭ������� ?
��3��Ϊ̽����������ԭ��,ͬѧ�Ǽ�������������ʵ��:
����H2O2��Һ�е������η�̪,��,����5��0��1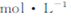 NaOH��Һ,��Һ�����Ѹ�ٱ���ɫ�Ҳ�������,10���Ӻ���Һ����ɫ,�ù�����������ЧӦ?
NaOH��Һ,��Һ�����Ѹ�ٱ���ɫ�Ҳ�������,10���Ӻ���Һ����ɫ,�ù�����������ЧӦ?
����0��1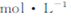 NaOH��Һ�е������η�̪��,��,��Һ���,10���Ӻ���Һ��ɫ�����Ա仯;�����Һ��ͨ������,��Һ��ɫ�����Ա仯?��ʵ���͢��У��ɵó��Ľ����� ?
NaOH��Һ�е������η�̪��,��,��Һ���,10���Ӻ���Һ��ɫ�����Ա仯;�����Һ��ͨ������,��Һ��ɫ�����Ա仯?��ʵ���͢��У��ɵó��Ľ����� ?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�̷���һ����Ҫ�Ļ���ԭ�ϡ�
������1���̷����壨FeSO4��7H2O�����ڱ��治�÷��ã����ױ������е��������������ʡ�Ϊ̽���̷���Ʒ�ı��������ij��ѧ��ȤС���ͬѧ���������ʵ�鷽����
ʵ������ȡ�����̷���Ʒ��������ˮ���ձ��������Һ��
��1������1��ȡ������Һ�����뼸�� �Լ���д��ѧʽ��������۲쵽����������Һ��Ѫ��ɫ��ʵ����ۣ�֤���̷���Ʒ�ѱ�������
��2������2��ȡ������Һ�����뼸������KMnO4��Һ������۲쵽�������� ��ʵ����ۣ�֤���̷���Ʒ��ȫ��������
��3��ʹ��FeSO4ʱ����Ҫ��ֹFe3+�ĸ��ţ����Լ����������۽��г��ӣ�д���������ۺ�����Ӧ�����ӷ���ʽ ��
��4��Ҫ��FeSO4��Һ�еõ��̷����壬������е�ʵ��������裺��������������ȴ�ᾧ�������ˡ���Ȼ�������һϵ�в�����û���õ������������������������˿�����ţ�
| A�������� | B��ʯ���� | C���ձ� | D�������� |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com