��һ����1����ͼΪ���������IJ��ֽṹ���е��������Ŵ�
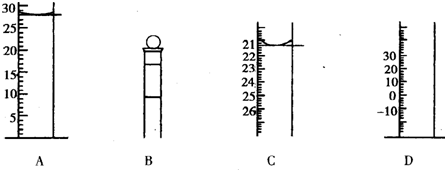
Aͼ��Һ����ʾ��Һ�����Ϊ
28.0
28.0
mL�����������������е�ij�ֲ���һҺ��������ƽ��ʱ����ΪN mL������ʱ����ΪM mL����M��N������ʹ�õ�������
C
C
������ĸ��ţ���
��2�����ξ��ᴿ��õ�NaCl��Һ���پ��������ᾧ����ɵþ��Σ�
������������ʹ�õ��Ĵ�������������Ϊ
������
������
��
�ڸ�ͬѧ�����þ��������Һ��������һʵ�飮ʵ������Ҫ��80mL l mol/L��NaCl��Һ�����ƹ�������������ƽ��ȡ�ľ�������Ϊ
5.9
5.9
g�����ڶ��ݵIJ��������Ĺ�������Ϊ
100mL����ƿ
100mL����ƿ
��
����������δ����ǩ���Լ�ƿ�зֱ�װ������ϡ��Һ��FeSO
4����H
2SO
4����BaCl
2����H
2O
2����Al��NO
3��
3����NaOH��
��1��ijͬѧ�벻�������Լ�����ͨ�����Թ�ȡ����������Һ�����������ʵ������Լ�ƿ������ȷ�ı�ǩ�����ܳɹ���
��
��
����ܡ����ܡ�����
��2��ʵ���з��֣���һ���ڻ��ʱ�������Լ��μӵ�˳��ͬ���������Բ�ͬ����������
�ݺ͢�
�ݺ͢�
����һ���ڻ��ʱ�������Լ��μӺ��ʱ�䲻ͬ���������Բ�ͬ����������
�ٺ͢�
�ٺ͢�
�������漰������ԭ��Ӧ�Ļ�ѧ����ʽΪ
4Fe��OH��2+O2+2H2O�T4Fe��OH��3
4Fe��OH��2+O2+2H2O�T4Fe��OH��3
��
��3�������ͬѧ����FeSO
4������������Fe
2+��ԭ�Ե�ʵ�飬ÿ�μ������������Ѽ�������ʵ���Һ�����ϣ���д������������Ӧ�����ӷ���ʽ
3Fe2++4H++NO3-�T3Fe3++NO��+2H2O
3Fe2++4H++NO3-�T3Fe3++NO��+2H2O
��
2Fe2++2H++H2O2�T2Fe3++2H2O
2Fe2++2H++H2O2�T2Fe3++2H2O
��
��4��ʵ����ʵ�������Ƶ�FeSO
4��Һ���ɳ��ڱ��棬���ڱ���ʱ����FeSO
4��Һ����ʱ��������������
��
��
�����ţ���ֹˮ�⣬�����ټ���
��ö����
��ö����
�����������ƣ�Ч������ã�






