
( 9��)A��B���Ƕ�����Ԫ�أ�ԭ�����������Ų�ʽ�ֱ�Ϊ(n��1)sx��nsx+1npx+3��A��B���γɻ�����D��D����ˮʱ�������ݳ�����������ʹ�����ǵ�ľ����ȼ����ش��������⣺
(1)�Ƚϵ����ܣ�I1(A)________I1(B)(�>����<������ͬ)���Ƚϵ縺�ԣ�A________B��
(2)ͨ��AԪ�صĻ��ϼ���________����AԪ�س������ּ�̬���н��ͣ�
����ԭ�ӽṹ�Ĺ۵���н��ͣ� _______________________________________��
���õ����ܵĹ۵���н��ͣ� ___________________________________________��
��д��D��ˮ��Ӧ�����ӷ���ʽ��_______________________________________��
( 9�� ) (1)��<����1�֣� ��<����1�֣� (2)��1�ۣ�1�֣�
������ԭ��ʧȥһ�����Ӻ��γ�1s22s22p6ʽ��ԭ�ӹ��ȫ�����ģ�1�������ӡ������ӽṹ��ϵ�����ͣ�������ʧȥ���ӣ�2�֣�
��Naԭ�ӵĵ�һ��������Խ�С���ڶ������ܱȵ�һ�����ܴ�ܶ��ͨ��Naԭ��ֻ��ʧȥһ������ ��2�֣���2Na2O2��2H2O===4Na����4OH����O2����2�֣�
����������������ݹ���ԭ����֪��s����������2�����ӣ�����x��1��D����ˮʱ�������ݳ�����������ʹ�����ǵ�ľ����ȼ������D�ǹ������ƣ���n��2������A���ƣ�B����Ԫ�ء�
��1���ǽ�����Խǿ����һ������Խ�������Ƶĵ�һ������С����Ԫ�صġ�ͬ���縺��Ҳ���Ƶ�С����Ԫ�صġ�
��2����������������1����ͨ���ԣ�1�ۡ�
��������ԭ��ʧȥ1�����Ӻ��γ�1s22s22p6ʽ��ԭ�ӹ��ȫ�����ģ�1�������ӡ������ӽṹ��ϵ�����ͣ�������ʧȥ���ӣ�����ͨ���ԣ�1�ۡ�
������Naԭ�ӵĵ�һ��������Խ�С�����ڶ������ܱȵ�һ�����ܴ�ܶ��ͨ��Naԭ��ֻ��ʧȥһ�����ӣ�����ԣ�1�ۡ�
�۹�����������ˮ�����������������ƣ���Ӧ�����ӷ���ʽ��2Na2O2��2H2O===4Na����4OH����O2����
���㣺���������ӵ��Ų��������ܼ��縺�Ե��й��ж�
���������ж�ԭ�Ӻ�����ӵ��Ų�ʱ��Ӧ�����ù���ԭ�����з��������Ȿ��Ĺؼ���������ʹ�����ǵ�ľ����ȼ���������������̶���һ�������ɡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
( 9��)A��B���Ƕ�����Ԫ�أ�ԭ�����������Ų�ʽ�ֱ�Ϊ(n��1)sx��nsx+1npx+3��A��B���γɻ�����D��D����ˮʱ�������ݳ�����������ʹ�����ǵ�ľ����ȼ����ش��������⣺
(1)�Ƚϵ����ܣ�I1(A)________I1(B)(�>����<������ͬ)���Ƚϵ縺�ԣ�A________B��
(2)ͨ��AԪ�صĻ��ϼ���________����AԪ�س������ּ�̬���н��ͣ�
����ԭ�ӽṹ�Ĺ۵���н��ͣ� _______________________________________��
���õ����ܵĹ۵���н��ͣ� ___________________________________________��
��д��D��ˮ��Ӧ�����ӷ���ʽ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧ����һ������ �ǽ������仯������ϰ���������棩 ���ͣ������
(9��)A��B��C��D��E������NH4Cl��Ba(OH)2��KCl��K2SO4��(NH4)2SO4��ɫ��Һ�е�һ�֣��������������ʱ�����������ǣ�
��A��B��Ϻ������ɫ���������Ⱥ�����������
��B��C���Ҳ������ɫ���������Ⱥ����������������ʹʪ��ĺ�ɫʯ����ֽ������
��B��E��Ϻ������������Ⱥ�Ҳ����ʹʪ��ĺ�ɫʯ����ֽ���������壮
��D���κ�һ����Һ��Ϻ������Ա仯�������������ش�
(1)A��________��B��________��C��________��D��___________��E��________(�û�ѧʽ��ʾ)��
(2)д���йط�Ӧ�����ӷ���ʽ��
A��B��________________________________________________________��
B��C��________________________________________________________��
B��E��_________________________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�人�н������Ƹ��и�����ѧ����ĩ���ԣ����ۣ���ѧ���� ���ͣ������
��14�֣�����A��E�������±���������ɵģ������¸����ʴ�1mLϡ�͵�1000mL��pH�ı仯��ͼ����ʾ������A��D��Ӧ�õ�E�� ��ش�
|
������ |
NH4+��H+��Na+ |
|
������ |
OH-��CH3COO-��Cl- |
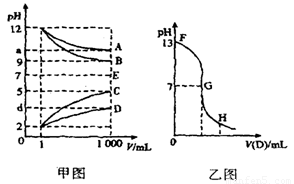
��1������pH�ı仯��ϵͼ�ף�д�����ʵĻ�ѧʽ��B�� ��D�� ��
��2��ͼ��a>9��������
��3����ȡŨ��ΪC1��B��Һ25mL����������εμ�0��2mol��L��D��Һ���ζ���������ҺpH�ı仯��������ͼ��ʾ��
��C1Ϊ ��
��G����Һ��������μ���D��Һ�����V 12��5mL����>��<��=��
�۳�����B��C��Һ��pH�ֱ���a��b��a+b=13��B��C���ǡ����ȫ��Ӧʱ������B��C��Һ�������VB��VC=
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��) A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬AԪ�ص�ԭ�Ӱ뾶��С��BԪ��ԭ�ӵ��������������ڲ��������2����CԪ��ԭ�ӵĵ��Ӳ���Ϊn������������Ϊ2n+l��A��B��C��Eÿ��Ԫ�ض�����DԪ��������ֻ��������ϵij�������� �ش��������⣺
��1��д�����и�Ԫ�ص����ƣ�C___________ E____________
��2����֪��BA3DA(g)+A2D(g)=BD2(g)+3A2(g) ��H= +49��0kJ��mol
BA3DA(g)+1��2D2(g)=BD2(g)+2A2(g) ��H = һ192��9kJ��mol
��д������̬BA3DA��ȫȼ��������̬A2D���Ȼ�ѧ����ʽ(���Ƴ���Ԫ�ط���д)
��3��![]() ��ȼ������A2������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��
��ȼ������A2������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��
![]()

��4����A��B��C��D����Ԫ�ؿ�����ɶ��ּ�����ǿ�ᷴӦ������ǿ�Ӧ�Ļ����������һ���ڼ����������ֽܷ����ɵ����ʵ��������ֲ���û������� ����һ�������������Ӫ�����ʣ�������Է�������Ϊ75���û������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com