
����Ŀ�����ʵ����Ǹ��л�ѧ���õ�������������������йؼ��㡣
��1����0.4 mol Al3����Al2(SO4)3��������SO42�������ʵ�����___________��
��2��___________molH2O2����ԭ������0.2molH3PO4����ԭ������ȡ�
��3��ij��������Һ�к���3.01��1022��Na+������Һ��SO42�������ʵ�����___________��
��4��0.7 mol H2O������Ϊ___________��
��5��483gNa2SO4��10H2O��������Na+�����ʵ�����___mol������H2O���ӵ���Ŀ��___����
��6��������ͬ��H2��NH3��SO2��O3���������У����з�����Ŀ���ٵ���____________��
��7��a��Xԭ�ӵ�������Ϊbg����X�����ԭ���������Ա�ʾΪ___________
��8������mgij���壬������ԭ�ӷ��ӣ���Ħ������ΪMg��mol-1���������ӵ�������NA��ʾ���������Ϸ��ż���Ӧ������д���пո�
�ٸ���������ʵ���Ϊ___________mol��
�ڸ���������ԭ������Ϊ___________����
���𰸡�0.6 mol0.40.025mol12.6g315NASO2bNA/am/M3mNA/M
��������
��1��Al2��SO4��3��n��Al3+����n��SO42-��=2��3���ʺ�0.4molAl3+��Al2��SO4��3������SO42-�����ʵ�����0.4mol��![]() =0.6mol���ʴ�Ϊ��0.6mol��
=0.6mol���ʴ�Ϊ��0.6mol��
��2��1��������Ӻ���8����ԭ�ӣ���0.2molH3PO4����1������������Ӻ���4����ԭ�ӣ�����1.6molԭ�ӵ�H2O2�����ʵ���Ϊ��![]() =0.4mol���ʴ�Ϊ��0.4��
=0.4mol���ʴ�Ϊ��0.4��
��3��ÿ�������ƻ�ѧʽ����������Ӻ������ӵĸ���֮��Ϊ1��2������3.01��1023��Na+�������Һ��SO42-�ĸ���Ϊ![]() ��3.01��1023������������ӵ����ʵ���n=
��3.01��1023������������ӵ����ʵ���n=![]() =0.25mol���ʴ�Ϊ��0.25mol��
=0.25mol���ʴ�Ϊ��0.25mol��
��4��0.7molˮ������Ϊ��18g/mol��0.7mol=12.6g���ʴ�Ϊ��12.6g��
��5��483gNa2SO410H2O�����ʵ���=![]() =1.5mol��Na+�����ʵ���ΪNa2SO410H2O��2��Ϊ1.5mol��2=3mol��H2O���ӵ����ʵ���ΪNa2SO410H2O��10��Ϊ1.5mol��10=15mol��������ˮ������ĿΪ15NA���ʴ�Ϊ��3��15NA��
=1.5mol��Na+�����ʵ���ΪNa2SO410H2O��2��Ϊ1.5mol��2=3mol��H2O���ӵ����ʵ���ΪNa2SO410H2O��10��Ϊ1.5mol��10=15mol��������ˮ������ĿΪ15NA���ʴ�Ϊ��3��15NA��
��6������n=![]() ��N=nNA��֪��������ȣ�Ħ������Խ����������ʵ���ԽС�����еķ�����Խ�٣����������У�Ħ�����������Ƕ����������Ժ��з��������ٵ��Ƕ������ʴ�Ϊ��SO2��
��N=nNA��֪��������ȣ�Ħ������Խ����������ʵ���ԽС�����еķ�����Խ�٣����������У�Ħ�����������Ƕ����������Ժ��з��������ٵ��Ƕ������ʴ�Ϊ��SO2��
��7��a��Xԭ�ӵ�������Ϊbg����NA��ԭ�ӵ�����ΪNA��![]() g����ֵ�ϵ��������ԭ���������ʸ�ԭ�����ԭ������Ϊ
g����ֵ�ϵ��������ԭ���������ʸ�ԭ�����ԭ������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(8)��m gij��������ʵ���Ϊ![]() =
=![]() mol���ʴ�Ϊ��
mol���ʴ�Ϊ��![]() ��
��
����Ϊһ�������к�����ԭ�ӣ����Ժ��е�ԭ����Ϊ��������2������Ϊ3��![]() mol��NAmol-1=
mol��NAmol-1=![]() NA���ʴ�Ϊ��
NA���ʴ�Ϊ��![]() NA��
NA��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������mgijX2���壬����Ħ������ΪM g/mol�������ӵ�������NA��ʾ����
��1������������ʵ���Ϊ________mol��
��2��һ��Xԭ�ӵ�����_________g��
��3���������ڱ�״���µ����Ϊ________L��
��4������������ˮ���γ�VL��Һ������Һ�����ʵ���Ũ��Ϊ________mol��L��1��
��5������������1Lˮ��(�����Ƿ�Ӧ)��������Һ���ܶ�Ϊ��g/cm3�������Һ�����ʵ���Ũ��Ϊ__________mol��L��1��
��6����ͬ״���£���X2�뵪���������1:4��ϣ��û��������������ܶ�Ϊ14.4��X2����Է�������Ϊ_______��
��7���������X2���ܶ�Ϊ1.25 g/L��Xԭ���������_____�����ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ĺ�ҵ�������Բ�������ͷ���Ҳ���Բ����л��ϳɷ������е�һ�ַ����DZ��ᷨ��
��.����ͷ�
![]()
��1����������Ƿ���ȫˮ�⣬����ѡ�õ�һ���Լ���__________������ţ���
a.��ˮ b.������Һ c.��� d.�⻯�ص�����Һ
��2����֪������������������¿�ת��Ϊ����(C3H6O3)��
��д����������������Һ��Ӧ�����ữ���л�����Ľṹ��ʽ��___________________��
��������Cu������ʱ�ɱ������ɱ�ͪ��(![]() )����������ʵ��֪����Ľṹ��ʽΪ_______��
)����������ʵ��֪����Ľṹ��ʽΪ_______��
��������һ�������·�Ӧ���ɸ߷��ӻ�����Ļ�ѧ����ʽΪ__________________________________
��������ŨH2SO4���������¿�������һ����ʹ��ˮ��ɫ���л���A�� A��һ�������¿��Ժϳ�һ�ֳ������л��߷��ӻ�����B��B�Ľṹ��ʽΪ____________________________��
��.����ϳɷ�

��3����ӦI�ķ�Ӧ������________�����з�Ӧ��ʱ����Ҫ��������Ҵ�����������Ŀ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�����õ����������(��Ҫ�ɷ�MnO2��MnO)�Ʊ�MnO2�Ĺ����������£�
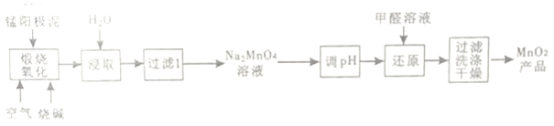
(1)������������ʱ��1mol MnO������ȫת��ΪNa2MnO4ʧȥ���ӵ����ʵ���Ϊ___________��MnO2���շ�Ӧ�Ļ�ѧ����ʽΪ__________________��
(2)����ȡ��ʱ��Ϊ���Na2MnO4�Ľ�ȡ�ʣ��ɲ�ȡ�Ĵ�ʩ��____________��____________(�о�2��)
(3)����pH���ǽ���ҺpH ����ԼΪ10����ֹpH�ϵ�ʱNa2MnO4��������������ԭ��Ӧ������MnO2��___________��д����pH��ֽ�ⶨ��ҺpH�IJ���_______________��
(4)����ԭ��ʱ���������������ɣ�������Ӧ�Ļ�ѧ����ʽΪ_____________��
(5)�ⶨ��Ʒ��MnO2���������IJ������£�
����1. ȷ��ȡmg��Ʒ������c1mol��L-1Na2C2O4��ҺV1mL (����)��������ϡ���ᣬˮԡ�������һ��ʱ�䡣(��֪��Na2C2O4+2H2SO4+MnO2=MnSO4+2CO2��+2H2O+Na2SO4)
����2. Ȼ����c2mol��L-1KMnO4����Һ�ζ�ʣ���Na2C2O4�ζ����յ�ʱ����KMnO4����ҺV2mL��(��֪��5H2C2O4+2KMnO4+3H2SO4=2MnSO4+10CO2��+K2SO4+8H2O)
����2��ζ��յ�ʱ�ж�������_____________����Ʒ��MnO2����������Ϊ��(MnO2)=____________(�г�����ı���ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. �����ӵ�������12g̼�������е�̼ԭ����
B. ��֪����������ͨ�������ӵ�����������������ʵ���
C. �����ӵ���������ֵ��6.02��1023
D. �����ӵ������ķ���ΪNA��ͨ����6.02��1023��ʾ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NAΪ�����ӵ�������ֵ������˵����ȷ����
A. ��״���£�2.24L������2.24L����C-H������Ϊ0.6NA
B. 16.8gFe��������ˮ�������ȳ�ַ�Ӧ��ת�Ƶ�����Ϊ0.8NA
C. 25Cʱ��1LpH=7��CH3COONH4��Һ�к�NH4+��һ��Ϊ1.0��10-7NA
D. 0.2molCO2��0.1molC���ܱ������г�ַ�Ӧ����CO������Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С��Ϊ��̽��SO2�����ʣ����������װ�ã�

ʵ�鲽�裺
�������Ӻ�װ�ã���������ԣ��ټ����Լ���
������A�Թ���
����ͭ˿���ϳ鶯�뿪Һ�档
��1��A�Թ��з�����Ӧ�Ļ�ѧ����ʽ��______��
��2��B�Թ��е�������______��
��3���Թ�C����������ijС��ȡһ���ַ�Ӧ�����Һ���ֱ�μ������Լ�������Ԥ���ܷ����ɳ����������ɳ�����д�����ɳ����Ļ�ѧʽ��
�����Լ� | �ܷ����ɳ��� | �����Ļ�ѧʽ |
��ˮ | _____________ | __________ |
��ˮ | __________ | ___________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������![]() ������ͭ��Һ�����²�����ȷ����
������ͭ��Һ�����²�����ȷ����![]() ����
����![]()
A. ��![]() �������500mL��Һ
�������500mL��Һ
B. ��![]() ������������ˮ�У�����ˮϡ����500mL
������������ˮ�У�����ˮϡ����500mL
C. ��ȡ![]() ����ͭ������500mLˮ
����ͭ������500mLˮ
D. ��![]()
![]() ��Һ�м���400mLˮ
��Һ�м���400mLˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺

��ѡ�Լ���Na2SO4��Һ��K2CO3��Һ��K2SO4��Һ������
��1�������ڵ�������______________���Լ�a��������_______________���ѧʽ��
��2�������Լ�b��������Ӧ�Ļ�ѧ����ʽΪ___________________________________��
��3���÷����ܲ��ܴﵽʵ��Ŀ�ģ�_____________�������ܣ�Ӧ��θĽ��������ܣ����ʲ��ûش�________________________________________��
��4���÷�����Ĺ���B����100mL 0.5mol/L����ҺB���������¿ɹ�ѡ���������
A.��ͷ�ι� B.��ƿ C.�ձ� D.ҩ�� E.��Ͳ F.������ƽ��
����������ƽ�Ƶù���B��������_________g��
��������ҺBʱ������������һ������Ҫʹ�õ���_____________������ĸ������ȱ�ٵ�������__________________________________��д�������ƣ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com