
N2(g)+2O2(g)![]() N2O4(g) ��H=+8.7 kJ��mol-1
N2O4(g) ��H=+8.7 kJ��mol-1
N2H4(g)+O2(g) ![]() N2(g)+2H2O(g) ��H=-534.0 kJ��mol-1
N2(g)+2H2O(g) ��H=-534.0 kJ��mol-1
���б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ���� ���� ��
A.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ��H=-542.7 kJ��mol-1
3N2(g)+4H2O(g) ��H=-542.7 kJ��mol-1
B.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ��H=-1 059.3 kJ��mol-1
3N2(g)+4H2O(g) ��H=-1 059.3 kJ��mol-1
C.N2H4(g)+![]() N2O4(g)
N2O4(g) ![]()
![]() N2(g)+2H2O(g) ��H=-1 076.7 kJ��mol-1
N2(g)+2H2O(g) ��H=-1 076.7 kJ��mol-1
D.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ��H=-1 076.7 kJ��mol-1
3N2(g)+4H2O(g) ��H=-1 076.7 kJ��mol-1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
N2(g)+2O2(g) �T�TN2O4(g)����H=+8.7 kJ��mol -1
N2H4(g)+O2(g) �T�TN2(g)+2H2O(g)����H=-534.0 kJ��mol -1???
���б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ����
A.2N2H4(g)+N2O4(g) �T�T3N2(g)+4H2O(g);��H=-542.7 kJ��mol -1??
B.2N2H4(g)+N2O4(g) �T�T3N2(g)+4H2O(g);��H=-1059.3 kJ��mol -1??
C.N2H4(g)+ ![]() N2O4(g) �T�T
N2O4(g) �T�T![]() N2(g)+2H2O(g);��H=-1076.7 kJ��mol -1??
N2(g)+2H2O(g);��H=-1076.7 kJ��mol -1??
D.2N2H4(g)+N2O4(g) �T�T3N2(g)+4H2O(g);��H=-1076.7 kJ��mol -1?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и���12���¿�У�����ϼ�⻯ѧ�Ծ��������棩 ���ͣ������
��(N2H4)�ǻ�����䳣�õ�ȼ�ϡ�
��1������ʵ���о����»�ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2����д�����Ʒ��Ļ�ѧ��Ӧ����ʽ____________________________������Ӧ��ת��0.2 mol���� ʱ�����Ƶ�Cu2O������Ϊ__________��
��2��һ������(N2H4)Ϊȼ�ϵĵ��װ����ͼ��ʾ����ȼ�ϵ�صĵ缫���ϲ��ö������ϣ�����ߵ缫��Ӧ���ڵ缫���������������ʹ������������Һ��ֽӴ����Կ����е�������Ϊ��������KOH��Һ��Ϊ����ʡ������Ϸ����ĵ缫��ӦΪ_________________�� �ڵ�ع���ʱ�����ĵ�����_______�缫�������غ�����________�缫�����ࡱ���Ҳࡱ����
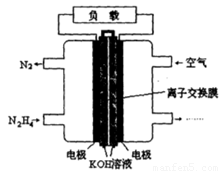
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ӱ�ʡ��һ��ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��(N2H4)�ǻ����������һ��ȼ�ϣ���ӦʱN2O4Ϊ������������N2��ˮ��������֪��
N2(g)+2O2(g)====N2O4(g) ��H="+8.7" kJ��mol-1
N2H4(g)+O2(g) ====N2(g)+2H2O(g) ��H="-534.0" kJ��mol-1
���б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ����
A��2N2H4(g)+N2O4(g) ====3N2(g)+4H2O(g) ��H="-542.7" kJ��mol-1
B��2N2H4(g)+N2O4(g) ====3N2(g)+4H2O(g)��H="-1059.3" kJ��mol-1
C��N2H4(g)+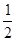 N2O4(g) ====
N2O4(g) ====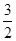 N2(g)+2H2O(g)��H="-1076.7" kJ��mol-1
N2(g)+2H2O(g)��H="-1076.7" kJ��mol-1
D��2N2H4(g)+N2O4(g) ====3N2(g)+4H2O(g)��H="-1076.7" kJ��mol-1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com