
ЁОЬтФПЁПЖЬжмЦкжїзхдЊЫиXЁЂYЁЂZЁЂMЕФдзгађЪ§вРДЮдіДѓЃЌXдзгКЫЭтзюЭтВуЕчзгЪ§ЪЧЦфЕчзгВуЪ§ЕФ2БЖЃЌXЁЂYЕФКЫЭтзюЭтВуЕчзгЪ§жЎБШЮЊ2ЃК3ЁЃН№ЪєЕЅжЪZдкY2жаШМЩеЩњГЩЕФЛЏКЯЮяПЩгыЫЎЗЂЩњбѕЛЏЛЙдЗДгІЁЃ![]() ЕФзюЭтВуЮЊ8ЕчзгНсЙЙЁЃЧыЛиД№ЯТСаЮЪЬтЃК.
ЕФзюЭтВуЮЊ8ЕчзгНсЙЙЁЃЧыЛиД№ЯТСаЮЪЬтЃК.
ЃЈ1ЃЉXЕФзюМђЕЅЧтЛЏЮяЕФЗжзгЪНЮЊ__________ЁЃ
ЃЈ2ЃЉZЕФзюИпМлбѕЛЏЮяЕФЫЎЛЏЮяЪєгк_________ЃЈЬюЁАЫсЁБЛђЁАМюЁБЃЉЁЃ
ЃЈ3ЃЉШШЮШЖЈадЃКXЕФзюМђЕЅЧтЛЏЮяБШYЕФзюМђЕЅЧтЛЏЮя_______ЃЈЬюЁАЧПЁБЛђЁАШѕЁБЃЉЁЃ
ЃЈ4ЃЉН№ЪєЕЅжЪZдкY2жаШМЩеЩњГЩЛЏКЯЮяЃЌ1molИУЛЏКЯЮягыЫЎЗДгІЪБзЊвЦЕчзгЪ§ЮЊ_______molЁЃ
ЃЈ5ЃЉYЁЂMСНдЊЫижЎМфаЮГЩЕФЛЏКЯЮяЃЌГЃгУзїЫЎЕФЯћЖОМСЕФЪЧ_______ЃЈЬюЗжзгЪНЃЉЁЃ
ЁОД№АИЁПCH4 Мю Шѕ 1 ClO2
ЁОНтЮіЁП
XдзгКЫЭтзюЭтВуЕчзгЪ§ЪЧЦфЕчзгВуЪ§ЕФ2БЖЃЌXЪЧCдЊЫиЃЛXЁЂYЕФКЫЭтзюЭтВуЕчзгЪ§жЎБШЮЊ2ЃК3ЃЌЫЕУїYЕФзюЭтВуЕчзгЪ§ЮЊ6ЃЌYЪЧOдЊЫиЃЛН№ЪєЕЅжЪZдкY2жаШМЩеЩњГЩЕФЛЏКЯЮяПЩгыЫЎЗЂЩњбѕЛЏЛЙдЗДгІЃЌЫЕУїZЪЧNaдЊЫиЃЛ![]() ЕФзюЭтВуЮЊ8ЕчзгНсЙЙЃЌЫЕУїMЪЧClдЊЫиЁЃ
ЕФзюЭтВуЮЊ8ЕчзгНсЙЙЃЌЫЕУїMЪЧClдЊЫиЁЃ
ЃЈ1ЃЉИљОнЗжЮіЃЌXЪЧCдЊЫиЃЌCЕФзюМђЕЅЧтЛЏЮяЕФЗжзгЪНЮЊCH4ЃЛ
ЃЈ2ЃЉИљОнЗжЮіЃЌZЪЧNaдЊЫиЃЌNaЕФзюИпМлбѕЛЏЮяЕФЫЎЛЏЮяЪЧNaOHЃЌЪЧЧПМюЃЛ
ЃЈ3ЃЉXЪЧCдЊЫиЃЌYЪЧOдЊЫиЃЌЗЧН№ЪєаддНЧПЃЌЧтЛЏЮядНЮШЖЈЃЌЗЧН№ЪєадCЃМOЃЌЙЪCH4ЕФШШЮШЖЈадШѕгкH2OЃЛ
ЃЈ4ЃЉNaдкO2жаШМЩеЩњГЩЕФNa2O2гыЫЎЗДгІ2Na2O2+2H2O=4NaOH+O2 ЁќЃЌNa2O2жа-1МлЕФOЗЂЩњЦчЛЏЃЌБфЮЊ0МлКЭ-2МлЃЌЙЪ1mol Na2O2гыЫЎЗДгІЪБзЊвЦЕчзгЪ§ЮЊ1molЃЛ
ЃЈ5ЃЉТШЕФбѕЛЏЮяжаЃЌClO2ГЃзїЮЊЫЎЕФЯћЖОМСЁЃ


 ЯАЬтОЋбЁЯЕСаД№АИ
ЯАЬтОЋбЁЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЯГІВн(Gelsemium)ЮЊжаЙњЙХДњОХДѓЖОвЉжЎвЛЃЌОнМЧдиФмЁАМћбЊЗтКэЁБЃЌЯжДњВщУїЫќЪЧКљТћЬйПЦжВЮяКљТћЬйЃЌЦфжаЕФЖОЫиКмЖрЃЌЯТСаЪЧЗжРыГіРДЕФЫФжжЖОЫиЕФНсЙЙЪНЃЌЯТСаЭЦЖЯе§ШЗЕФЪЧ

A. ЂйЁЂЂкЁЂЂлЁЂЂмЛЅЮЊЭЌЗжвьЙЙЬх
B. ЂйЁЂЂлЛЅЮЊЭЌЯЕЮя
C. ЕШЮяжЪЕФСПЕФЂкЁЂЂмЗжБ№дкзуСПбѕЦјжаЭъШЋШМЩеЃЌЧАепЯћКФбѕЦјБШКѓепЩй
D. ЂйЁЂЂкЁЂЂлЁЂЂмОљФмгыЧтбѕЛЏФЦШмвКЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНёгавЛЛьКЯЮяЕФЫЎШмвКЃЌжЛПЩФмКЌгавдЯТРызгжаЕФШєИЩжжЃКK+ЁЂAl3+ЁЂFe3+ЁЂMg2+ЁЂBa2+ЁЂNH4+ЁЂClЁЂCO32ЁЂSO42ЃЌЯжШЁШ§ЗнИї100mLШмвКНјааШчЯТЪЕбщЃК
ЂйЕквЛЗнМгШыAgNO3ШмвКгаГСЕэВњЩњ
ЂкЕкЖўЗнМгЙ§СПNaOHШмвКМгШШКѓЃЌжЛЪеМЏЕНЦјЬх0.02molЃЌзюжеЮоГСЕэЩњГЩЃЌЭЌЪБЕУЕНШмвКМз
ЂлдкМзШмвКжаЭЈШыЙ§СПCO2ЃЌЩњГЩАзЩЋГСЕэЃЌГСЕэОЙ§ТЫЁЂЯДЕгЁЂзЦЩеЃЌжЪСПЮЊ1.02g
ЂмЕкШ§ЗнМгзуСПBaCl2ШмвККѓЃЌЕУАзЩЋГСЕэЃЌГСЕэОзуСПбЮЫсЯДЕгЁЂИЩдяКѓЃЌжЪСПЮЊ11.65g
ЯТСаЫЕЗЈВЛе§ШЗЕФЪЧЃЈ ЃЉ
A.дШмвКжавЛЖЈВЛДцдкЕФРызгЮЊFe3+ЁЂMg2+ЁЂCO32ЁЂBa2+
B.c(SO42)=0.5mol/L
C.ЮоЗЈХаЖЯдШмвКжаЪЧЗёДцдкCl
D.ЮоЗЈХаЖЯдШмвКжаЪЧЗёДцдкK+
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТБэЪЧдЊЫижмЦкБэЕФвЛВПЗжЃЌБэжаЕФУПИізжФИДњБэвЛжждЊЫиЃЌЧыИљОнвЊЧѓЛиД№ЮЪЬтЁЃ
зх жмЦк | ЂёA | 0 | |||||||
1 | a | ЂђA | ЂѓA | ЂєA | ЂѕA | ЂіA | ЂїA | ||
2 | b | c | d | ||||||
3 | e | f | g |
ЃЈ1ЃЉдЊЫиgдкдЊЫижмЦкБэЕФЮЛжУЮЊ____________________ЁЃ
ЃЈ2ЃЉbКЭgСНжждЊЫиЕФдзгАыОЖДѓаЁЙиЯЕЃКb______gЃЈЬюЁА>ЁБЛђЁА<ЁБЃЉ.
ЃЈ3ЃЉгЩдзгИіЪ§БШЮЊ1ЃК1ЃК1ЕФaЁЂbЁЂcШ§жждЊЫизщГЩЕФЙВМлЛЏКЯЮяXЃЌЙВаЮГЩ4ЖдЙВгУЕчзгЖдЃЌдђXЕФНсЙЙЪНЮЊ______________ЁЃ
ЃЈ4ЃЉfЕФзюИпМлбѕЛЏЮягыeЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮядкШмвКжаЗДгІЕФРызгЗНГЬЪНЮЊ_________________________ЁЃ
ЃЈ5ЃЉAЁЂBЁЂDЁЂEЪЧгЩЩЯЪіВПЗждЊЫизщГЩЕФЛЏКЯЮяЃЌЫќУЧжЎМфЕФзЊЛЏЙиЯЕШчЭМЫљЪОЃЈВПЗжВњЮявбТдШЅЃЉЁЃAЁЂBЁЂDЕФбцЩЋЗДгІОљГЪЛЦЩЋЃЌЫЎШмвКОљЮЊМюадЁЃЧыЛиД№ЃК
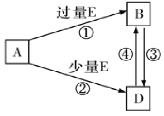
ЂйEЕФЕчзгЪНЮЊ_______________ЃЌBЕФЛЏбЇЪНЮЊ____________________ЁЃ
ЂкAжаЕФЛЏбЇМќРраЭЮЊ____________________
ЂлздШЛНчжаДцдкBЁЂDКЭH2OАДвЛЖЈБШР§НсОЇЖјГЩЕФЙЬЬхЁЃШЁвЛЖЈСПИУЙЬЬхШмгкЫЎХфГЩ100mLШмвКЃЌВтЕУШмвКжаН№ЪєбєРызгЕФХЈЖШЮЊ0.5mo/LЁЃШєШЁЯрЭЌжЪСПЕФЙЬЬхМгШШжСжЪСПВЛдйЗЂЩњБфЛЏЃЌЪЃгрЙЬЬхЕФжЪСПЮЊ___________gЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкЭЈЗчГїжаНјааЯТСаЪЕбщЃК
ЪЕбщ ВНжш |
|
|
|
|
ЯжЯѓ | FeБэУцВњЩњДѓСП ЮоЩЋЦјЬхЃЌдквКУц ЩЯЗНБфКьзиЩЋ | FeБэУцЮоУї ЯдБфЛЏ | ГЃЮТЯТгаЮоЩЋЦјХнЃЌМгШШКѓЃЌCuБэУцВњЩњЮоЩЋЦјЬхЃЌдквКУцЩЯЗНБфКьзиЩЋ | CuБэУцВњЩњКь зиЩЋЦјЬх |
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЂйжаЦјЬхгЩЮоЩЋБфЮЊКьзиЩЋЃЌКьзиЩЋЦјЬхЪЧ__________________ЃЈЬюЗжзгЪНЃЉЁЃ
ЃЈ2ЃЉЂкжаЕФЯжЯѓЫЕУїFeЗЂЩњСЫ______ЯжЯѓЃЌВњЩњЕФдвђЪЧ________________________ЁЃ
ЃЈ3ЃЉЖдБШЂйЂкжаЕФЯжЯѓЃЌ______ЃЈЬюЁАФмЁБЛђЁАВЛФмЁБЃЉЫЕУїЯЁЯѕЫсЕФбѕЛЏадЧПгкХЈЯѕЫсЁЃ
ЃЈ4ЃЉЖдБШЂлЂмжаЕФЯжЯѓЃЌЫЕУїбѕЛЏадЃКЯЁЯѕЫс______ЃЈЬюЁА>ЁБЛђЁА<ЁБЃЉХЈЯѕЫсЁЃ
ЃЈ5ЃЉЂлжадкМгШШЪБЕФЛЏбЇЗДгІЗНГЬЪНЮЊ________________________ЃЌДЫЗДгІжаЯЁЯѕЫсГ§БэЯжГібѕЛЏадЭтЃЌЛЙБэЯжГі____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖэТоЫЙгУЁАжЪзгЁЊMЁБКХдЫдиЛ№М§ГЩЙІНЋЁАЙтЯпЁБКХЮРаЧЫЭШыдЄЖЈЙьЕРЃЌЗЂЩфгУЕФдЫдиЛ№М§ЪЙгУЕФЪЧвдвКЧтЮЊШМЩеМСЃЌвКбѕЮЊбѕЛЏМСЕФИпФмЕЭЮТЭЦНјМСЃЌвбжЊЃК
ЃЈ1ЃЉH2(g)=H2(l) ЁїH1=-0.92kJЁЄmol-1
ЃЈ2ЃЉO2(g)=O2(l) ЁїH2=-6.84kJЁЄmol-1
ЃЈ3ЃЉШчЭМЃК
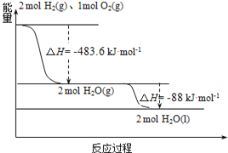
ЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A. 2mol H2(g)гы1molO2(g)ЫљОпгаЕФзмФмСПБШ2molH2O(g)ЫљОпгаЕФзмФмСПЕЭ
B. Л№М§жавКЧтШМЩеЕФШШЛЏбЇЗНГЬЪНЮЊЃК2H2(l)+O2(l)=2H2O(g) ЁїH=-474.92kJЁЄmol-1
C. ЧтЦјЕФШМЩеШШЮЊЁїH=-241.8kJЁЄmol-1
D. H2O(g)БфГЩH2O(l)ЕФЙ§ГЬжаЃЌЖЯМќЮќЪеЕФФмСПаЁгкГЩМќЗХГіЕФФмСП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЂё.ЃЈ1ЃЉ298KЪБЃЌ0.5 mol C2H4 (g)ЭъШЋШМЩеЩњГЩЖўбѕЛЏЬМКЭвКЬЌЫЎЃЌЗХГі705.5kJЕФШШСПЁЃЧыаДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪН______________________ЁЃ
ЃЈ2ЃЉNa2SO3ОпгаЛЙдадЃЌЦфЫЎШмвКПЩвдЮќЪеCl2(g)ЃЌМѕЩйЛЗОГЮлШОЁЃ
вбжЊЗДгІЃК Na2SO3(aq)+Cl2(g)+H2O(l)ЃНNa2SO4(aq)+2HCl(aq) ІЄH1=a kJЁЄmol1
Cl2(g)+H2O(l)ЃНHCl(aq)+HClO(aq) ІЄH2=b kJЁЄmol1
ЪдаДГіNa2SO3(aq)гыHClO(aq)ЗДгІЕФШШЛЏбЇЗНГЬЪНЃК________________________ЁЃ
Ђђ.КьЗЏФЦЃЈNa2Cr2O7ЁЄ2H2OЃЉЪЧживЊЕФЛЏЙЄдСЯЃЌЙЄвЕЩЯгУИѕЬњПѓЃЈжївЊГЩЗжЪЧFeOЁЄCr2O3ЃЉжЦБИКьЗЏФЦЕФЙ§ГЬжаЛсЗЂЩњШчЯТЗДгІЃК4FeO(s)ЃЋ4Cr2O3(s)ЃЋ8Na2CO3(s)ЃЋ7O2(g) ![]() 8Na2CrO4(s)ЃЋ2Fe2O3(s)ЃЋ8CO2(g)ЁЁІЄH<0
8Na2CrO4(s)ЃЋ2Fe2O3(s)ЃЋ8CO2(g)ЁЁІЄH<0
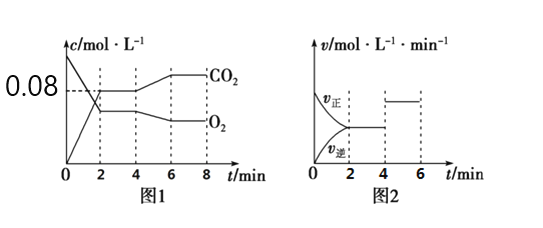
ЃЈ1ЃЉЧыаДГіЩЯЪіЗДгІЕФЛЏбЇЦНКтГЃЪ§БэДяЪНЃКKЃН_____________________ЁЃ
ЃЈ2ЃЉШчЭМ1ЫљЪОЃЌдк0~2minФкCO2ЕФЦНОљЗДгІЫйТЪЮЊ____________________ЁЃ
ЃЈ3ЃЉЭМ1ЁЂЭМ2БэЪОЩЯЪіЗДгІдк2minЪБДяЕНЦНКтЁЂдк4minЪБвђИФБфФГИіЬѕМўЖјЗЂЩњБфЛЏЕФЧњЯпЁЃгЩЭМ1ХаЖЯЃЌЗДгІНјаажС4minЪБЃЌЧњЯпЗЂЩњБфЛЏЕФдвђЪЧ______________ЃЈгУЮФзжБэДяЃЉЃЛгЩЭМ2ХаЖЯЃЌ4minЕН6minЕФЧњЯпБфЛЏЕФдвђПЩФмЪЧ________ЃЈЬюаДађКХЃЉЁЃ
a.Щ§ИпЮТЖШ bЃЎМгДпЛЏМС cЃЎЭЈШыO2 dЃЎЫѕаЁШнЦїЬхЛ§
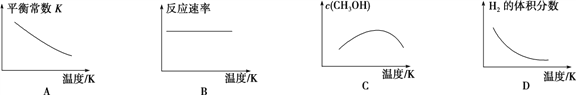
ЃЈ4ЃЉЙЄвЕЩЯПЩгУЩЯЪіЗДгІжаЕФИБВњЮяCO2РДЩњВњМзДМЃКCO2(g)ЃЋ3H2(g) ![]() CH3OH(g)ЃЋH2O(g)ЁЃ
CH3OH(g)ЃЋH2O(g)ЁЃ
вбжЊИУЗДгІФмздЗЂНјааЃЌдђЯТСаЭМЯёе§ШЗЕФЪЧ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШ§ТШЧтЙшЃЈSiHCl3ЃЉЪЧжЦБИЙшЭщЁЂЖрОЇЙшЕФживЊдСЯЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉSiHCl3дкГЃЮТГЃбЙЯТЮЊвзЛгЗЂЕФЮоЩЋЭИУївКЬхЃЌгіГБЦјЪБЗЂбЬЩњГЩ(HSiO)2OЕШЃЌаДГіИУЗДгІЕФЛЏбЇЗНГЬЪН__________ЁЃ
ЃЈ2ЃЉSiHCl3дкДпЛЏМСзїгУЯТЗЂЩњЗДгІЃК
2SiHCl3(g)![]() SiH2Cl2(g)+ SiCl4(g) ІЄH1=48 kJЁЄmol1
SiH2Cl2(g)+ SiCl4(g) ІЄH1=48 kJЁЄmol1
3SiH2Cl2(g)![]() SiH4(g)+2SiHCl3 (g) ІЄH2=30 kJЁЄmol1
SiH4(g)+2SiHCl3 (g) ІЄH2=30 kJЁЄmol1
дђЗДгІ4SiHCl3(g)![]() SiH4(g)+ 3SiCl4(g)ЕФІЄH=__________ kJЁЄmol1ЁЃ
SiH4(g)+ 3SiCl4(g)ЕФІЄH=__________ kJЁЄmol1ЁЃ
ЃЈ3ЃЉЖдгкЗДгІ2SiHCl3(g)![]() SiH2Cl2(g)+SiCl4(g)ЃЌВЩгУДѓПзШѕМюадвѕРызгНЛЛЛЪїжЌДпЛЏМСЃЌдк323 KКЭ343 KЪБSiHCl3ЕФзЊЛЏТЪЫцЪБМфБфЛЏЕФНсЙћШчЭМЫљЪОЁЃ
SiH2Cl2(g)+SiCl4(g)ЃЌВЩгУДѓПзШѕМюадвѕРызгНЛЛЛЪїжЌДпЛЏМСЃЌдк323 KКЭ343 KЪБSiHCl3ЕФзЊЛЏТЪЫцЪБМфБфЛЏЕФНсЙћШчЭМЫљЪОЁЃ
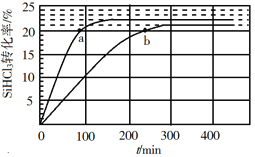
Ђй343 KЪБЗДгІЕФЦНКтзЊЛЏТЪІС=_________%ЁЃЦНКтГЃЪ§K343 K=__________ЃЈБЃСє2ЮЛаЁЪ§ЃЉЁЃ
Ђкдк343 KЯТЃКвЊЬсИпSiHCl3зЊЛЏТЪЃЌПЩВЩШЁЕФДыЪЉЪЧ___________ЃЛвЊЫѕЖЬЗДгІДяЕНЦНКтЕФЪБМфЃЌПЩВЩШЁЕФДыЪЉга____________ЁЂ___________ЁЃ
ЂлБШНЯaЁЂbДІЗДгІЫйТЪДѓаЁЃКІдa________ІдbЃЈЬюЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБЃЉЁЃЗДгІЫйТЪІд=Іде§ІдФц=![]()
![]() ЃЌkе§ЁЂkФцЗжБ№ЮЊе§ЁЂФцЯђЗДгІЫйТЪГЃЪ§ЃЌxЮЊЮяжЪЕФСПЗжЪ§ЃЌМЦЫуaДІ
ЃЌkе§ЁЂkФцЗжБ№ЮЊе§ЁЂФцЯђЗДгІЫйТЪГЃЪ§ЃЌxЮЊЮяжЪЕФСПЗжЪ§ЃЌМЦЫуaДІ![]() =__________ЃЈБЃСє1ЮЛаЁЪ§ЃЉЁЃ
=__________ЃЈБЃСє1ЮЛаЁЪ§ЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП25ЁцЪБЃЌЯђХЈЖШОљЮЊ0.1molЁЄL-1ЁЂЬхЛ§ОљЮЊ100mLЕФСНжжвЛдЊЫсHXЁЂHYШмвКжаЗжБ№МгШыNaOHЙЬЬхЃЌШмвКжа![]() Ыцn(NaOH)ЕФБфЛЏШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
Ыцn(NaOH)ЕФБфЛЏШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ

A.HXЮЊШѕЫсЃЌHYЮЊЧПЫс
B.ЫЎЕФЕчРыГЬЖШЃКb>c>d
C.CЕуЖдгІЕФШмвКжаЃКc(HY)>c(Y-)
D.ШєНЋcЕугыdЕуЕФШмвКШЋВПЛьКЯЃЌШмвКжаРызгХЈЖШДѓаЁЃКc(Na+)>c(X-)>c(Y-)>c(H+)>c(OH-)
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com