

| �۵�/�� | �е�/�� | ��ע | |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ���л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |
���� ��1����ͼ��a����֪���������ϣ�Լռ��ʯʹ�����ı���Ϊ��4%+96%��85%��80%=69%��
��2������ʯΪԭ�ϣ��ù����������ܽ�Ca5F��PO4��3���Ƶ����ᣬ���������غ���д��ѧ����ʽ������PԪ���غ�ɵù�ϵʽP2O5��2H3PO4�����ݴ˹�ϵʽ���㣻
��3����SiO2��������̿����ʯ��ϣ����³��˷�Ӧ���ɰ���֮�⣬�õ��������Թ�����CaSiO3��������ȴ��1��2���¶�������۵�ȽϷ�������״̬��
��4�����������HF��Ӧ�����ķ��������壬�����Ľ�̿����ȫȼ������CO�������β������Ҫ����SiF4��CO��������������PH3��H2S��HF�ȣ���β��ͨ�봿����Һ��SiF4��HF��H2S��̼���Ʒ�Ӧ����ȥ�����������ǿ�����ԣ��ɳ���ǿ��ԭ�Ե�PH3��
��5�������ʪ�����ᣬ�ȷ����Ṥ�����ò�Ʒ���ȴ�
��� �⣺��1����ͼ��a����֪���������ϣ�Լռ��ʯʹ�����ı���Ϊ��4%+96%��85%��80%=69%��
�ʴ�Ϊ��69��
��2������ʯΪԭ�ϣ��ù����������ܽ�Ca5F��PO4��3���Ƶ����ᣬ���������غ㶨�ɿɵ÷�Ӧ�Ļ�ѧ����ʽΪCa5F��PO4��3+5H2SO4=3H3PO4+5CaSO4+HF����
����PԪ���غ�ɵù�ϵʽP2O5��2H3PO4��142��P2O5����ȡ196�����ᣬ1t�ۺϺ���P2O5Լ30%����ʯ������P2O5������Ϊ0.3t�����Կ��Ƶõ�85%����Ʒ���������Ϊ$\frac{196��0.3t}{142��85%}$=0.49t��
�ʴ�Ϊ��Ca5F��PO4��3+5H2SO4=3H3PO4+5CaSO4+HF����0.49��
��3����SiO2��������̿����ʯ��ϣ����³��˷�Ӧ���ɰ���֮�⣬�õ��������Թ�����CaSiO3����ȴ��1���¶���70�棬280.5�棾t��44�棬���Դ�ʱ��Ҫ�ij�������Һ̬���ף���ȴ��2���¶���18�棬���ڰ����۵㣬�ʴ�ʱ����Ҫ�������ǹ�����ף�
�ʴ�Ϊ��CaSiO3��Һ̬���ף���̬���ף�
��4�����������HF��Ӧ�����ķ��������壬�����Ľ�̿����ȫȼ������CO�������β������Ҫ����SiF4��CO��������������PH3��H2S��HF�ȣ���β��ͨ�봿����Һ��SiF4��HF��H2S��̼���Ʒ�Ӧ����ȥ�����������ǿ�����ԣ��ɳ���ǿ��ԭ�Ե�PH3��
�ʴ�Ϊ��SiF4��CO��SiF4��H2S��HF��PH3��
��5�������ʪ�����ᣬ�ȷ����Ṥ�ո��ӣ��ܺĸߣ��������ò�Ʒ���ȴ������٣���������ã�
�ʴ�Ϊ����Ʒ���ȸߣ�
���� ���⿼�����Ʊ���������ƣ���Ŀ�Ѷ��еȣ��漰��ʯ����Ҫ��;����Ӧԭ�����йؼ��㣬�����Ǹ߿��еij������ͣ������ۺ���ǿ�����ض�ѧ���������⡢������������������ͽ��ⷽ����ָ������ȷʵ��Ŀ�ġ�ʵ��ԭ��Ϊ���ؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
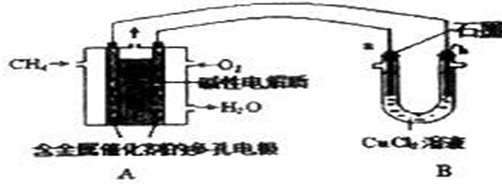
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧʵ�����е����Ǵ��Ⱥܸߣ�������ʳ�� | |
| B�� | ʵ��������ȡ���ж����壬����ͨ�缴�� | |
| C�� | Ϊ�˽�Լ��ˮ��ʵ�������ù��ķ�ϴҺ������������ϴ���� | |
| D�� | �������ֽӴ�ҩƷ����Ҫ�ѱǿ״յ�������ȥ��ҩƷ����ζ�����ó��κ�ҩƷ��ζ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu����Ũ���ᷴӦ����������Ũ���ᷴӦ | |
| B�� | Cu��Ũ���ᷴӦ����ϡ���ᷴӦ�� | |
| C�� | N2��O2�ڳ��¡���ѹ�²���Ӧ���ŵ�ʱ�ɷ�Ӧ | |
| D�� | Fe��Ũ���ᷴӦ����ϡ���ᷴӦ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
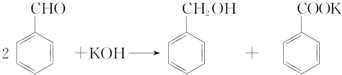
| �� Һ | ��������� | ����� |
| ��1������ʯ��ˮ | CO2 | |
| ��2��AlCl3��Һ | ����NH3 | |
| ��3��������NaOH��NaAlO2 | ����CO2 | |
| ��4��������NaOH��NaAlO2 | ��μ�ϡ���� | |
| ��5��MgCl2��AlCl3���Һ | ��μ�NaOH������ | |
| ��6��NaOH��Һ | ��μ�AlCl3������ |
�鿴�𰸺ͽ���>>
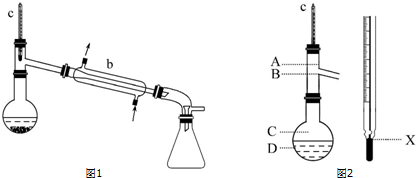
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ��״ | �۵�/�� | �е�/�� | �ܶ�/g•cm-3 | ˮ���� |
| ����ȩ | ��ɫҺ�壬������ζ | -26 | 178.1 | 1.041 5 | �� |
| ������ | ��ɫƬ״����״���� | 122.4 | 248 | 1.265 9 | �� |
| ���״� | ��ɫҺ�壬�з���ζ | -15.3 | 205.35 | 1.041 9 | �� |
| ���� | ��ɫ��Һ�壬 ������̼�����ζ | -116.3 | 34.6 | 0.71 | ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com