
����Ŀ����NAΪ�����ӵ�������ֵ������˵������ȷ���ǣ� ��
A.һ�������£��ϳɰ���Ӧ����0.5molN2������Ӧ����Ӧ��ת�Ƶ�����Ϊ3NA
B.10�˻�������2-�����Ļ��Һ�У�̼Ԫ�ص���������Ϊ72%����������������ԭ����ĿΪ![]() NA
NA
C.����ͭ�뺬0.4molHNO3��Ũ���ᷴӦ����Ӧ�е���ת����Ϊ0.2NA
D.���³�ѹ����28g�����辧�壬������������Si��Si����ĿΪ2NA
���𰸡�C
��������
A����Ӧ��N2ת��ΪNH3����Ԫ����0�۱�Ϊ-3�ۣ���1mol N2���뷴Ӧת��6mol���ӣ���0.5molN2������Ӧ����Ӧ��ת�Ƶ�����Ϊ3mol���ӣ�����Ϊ3 NA����A��ȷ��
B��������ķ���ʽΪC6H12��2-�����ķ���ʽΪC3H8O���൱��C3H6��H2O��̼Ԫ�ص���������Ϊ72%������![]() �����10�˻��Һ�к�CH2������Ϊ8.4g������H2O��Ϊ1.6g�������к��е���ԭ����ĿΪ
�����10�˻��Һ�к�CH2������Ϊ8.4g������H2O��Ϊ1.6g�������к��е���ԭ����ĿΪ![]() NA=
NA=![]() NA����B��ȷ��
NA����B��ȷ��
C������ͭ�뺬0.4 mol HNO3��Ũ���ᷴӦ����ֻ����NO2��������ӦΪCu+4HNO3(Ũ)= Cu(NO3)2+2H2O+2NO2������Ԫ����+5�۱�Ϊ+4�ۣ�0.4molŨHNO3������뷴Ӧ������0.2mol����ԭ������ת����ĿΪ0.2NA�������ŷ�Ӧ������Ũ�����ϡ������������NO2��ΪNO����Ԫ����+5�۱�Ϊ+2�ۣ�ת�Ƶ�����Ŀ���࣬�����ת����ĿΪ����0.2 NA����C����
D����������һ��Siԭ��ƽ����������Si��Si�������³�ѹ����28g�����辧��Ϊ1mol��������������Si��Si����ĿΪ2NA����D��ȷ��
��ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о������к�����(��Ҫ��SO2��H2S)��ת��������Ҫ���塣
��1����ʪ��������д��������SO2ת��ΪHSO3-������ʽ�� ��
��2�������е��������������H2S��������Ӧ������SO42-��������Ӧ�������仯ʾ��ͼ������
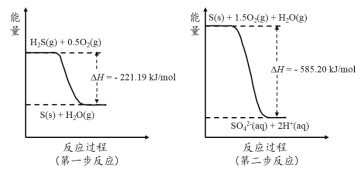
1mol H2S(g)ȫ��������SO42-(aq)������ѧ����ʽΪ ��
��3���������������ӽ���Ĥȼ�ϵ�ؿ������ô�������SO2������������װ��ʾ��ͼ���£�
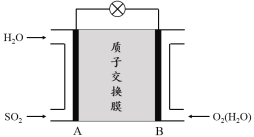
�� ��������������Ϊ ������A��B��������B��A������
�� �������缫��ӦʽΪ ��
��4��ȼú���������������Ǽ�����������������Ⱦ���ؼ���SO2�����ѳ���һ����ҵ�������£�
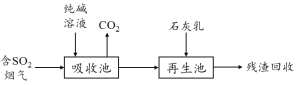
�ô�����Һ����SO2����ת��ΪHSO3-����Ӧ�����ӷ���ʽ�� ��
��ʯ��������������������Ż����ճأ����п���������SO2�������Ļ�ѧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���¹���ѧ�ҹ���(F.Haber)��1902�꿪ʼ�о��ɵ���������ֱ�Ӻϳɰ����ϳɰ�Ϊ����������ʳ������������Ҫ���ס���ԭ��ΪN2(g)+3H2(g)2NH3(g) ��H=-92.4kJ/mol
(1)����֪H-H���ļ���Ϊ436.0kJ/mol��N-H�ļ���Ϊ390.8kJ/mol����N![]() N�ļ���ԼΪ_____kJ/mol
N�ļ���ԼΪ_____kJ/mol
(2)�ϳɰ���Ӧ���Ӵ������ѷ�������������ý���������˷�Ӧ�����˻�ܡ�������ԭ����ͼ��ʾ������˴�����������Ӧ��������______����(������һ����Ӧ���������ڶ�����Ӧ��)��δʹ�ô���ʱ�淴Ӧ���______����Ӧ���(������������С����������������)
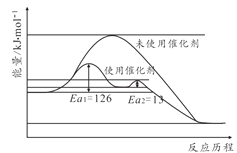
(3)��ƽ������ʽǶȿ��ǣ���ҵ������ȡ20MPa��50MPa�ĸ�ѹ�ϳɰ�ԭ��______
(4)һ���¶��º��������У��Բ�ͬ��H2��N2���ʵ���֮�ȼ��룬ƽ��ʱNH3���������ͼ��ʾ����H2ת����a��______b��(��"��������С����������������)������ʼѹǿΪ20MPa����b��ʱ��ϵ����ѹǿԼΪ______MPa��
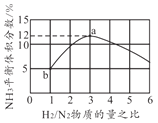
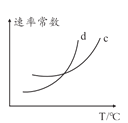
(5)���÷�Ӧ�����淴Ӧ���ʷֱ��ʾΪv��=K��![]() ��v��=K��c2(NH3)����һ���¶��£��÷�Ӧ ��ƽ�ⳣ��K=______(�ú�K����K���ı���ʽ��ʾ)����K����K�������¶ȵĺ����������¶����߶����ߣ���ͼ��c��d�ֱ��ʾ______��______���¶ȱ仯����(��K������K��)��
��v��=K��c2(NH3)����һ���¶��£��÷�Ӧ ��ƽ�ⳣ��K=______(�ú�K����K���ı���ʽ��ʾ)����K����K�������¶ȵĺ����������¶����߶����ߣ���ͼ��c��d�ֱ��ʾ______��______���¶ȱ仯����(��K������K��)��
(6)�����£���20mL��0.1mol/L��������ͨ��һ����������Ӧ����Һ������(������Һ����仯���Բ���)��������Һ��c(NH4+)=_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����о����ְ�����Ϣ���У�![]() (2��4-����-1-��ϩ)��
(2��4-����-1-��ϩ)��![]() (3��7-����-1-��ϩ)���Ҳϵ�����Ϣ��Ϊ��CH3(CH2)8CH==CH��CH==CH(CH2)8CH3������˵������ȷ����
(3��7-����-1-��ϩ)���Ҳϵ�����Ϣ��Ϊ��CH3(CH2)8CH==CH��CH==CH(CH2)8CH3������˵������ȷ����
A��2��4-����1��ϩ�ķ���ʽΪC9H18
B��2��4-����-1-��ϩ��3��7-����-1-��ϩ��Ϊͬϵ��
C������������Ϣ�ؾ���ʹ������Ȼ�̼��Һ��ɫ
D��1 mol�Ҳϵ�����Ϣ����1 mol Br2�ӳɣ�����ֻ��һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.ij���Ľṹ��ʽΪ
![]()
��1������������Ϊ_______________��
��2������������ϩ������һ��̼̼˫�����������ӳɶ�������ϩ���Ľṹ��_____�֣�����������ijȲ������һ��̼̼�����������������ӳɶ�������Ȳ���Ľṹ��ʽΪ_______________��
II����״���£�1.68 L��ɫ��ζ�Ŀ�ȼ��������������������ȫȼ�գ���������ͨ����������ʯ��ˮ���õ��İ�ɫ��������Ϊ15.0 g������������ʯ������ȼ�ղ������9.3 g��
��1������ȼ�ղ�����ˮ������Ϊ_________��
��2����ԭ�����ǵ�һ���������壬ͨ�������ƶ����ķ���ʽ��_____________��
��3����ԭ���������ֵ����ʵ�������̬���Ļ�����д�����ǵĽṹ��ʽ(Ҫ��д��һ��������������ʵĽṹ��ʽ����)��__________________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̫����Ϊ��Դ�ֽ�Fe3O4�����Ȼ�ѧ����������ѭ���ֽ�ˮ�ƱȵĹ�����ͼ��ʾ������������ȷ���ǣ� ��

A.H2�ı�ȼ���ȡ�H��-(��H1+��H2)
B.����I�ʵ����ͷ�Ӧ��ϵ�¶ȣ���Ӧ������
C.����II��3mo1FeO(s)������������lmolFe3O4(s)
D.������������������������������ѭ����H2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ�����
(1)1mol Na2O2������ˮ��ȫ��Ӧʱת�Ƶĵ�����_____________����Ӧ�����ӷ���ʽΪ________________��
(2)��ҵ���ɻ�ͭ������ͭ����Ҫ��ӦΪ��Cu2S+O2![]() 2Cu+SO2���÷�Ӧ�б���ԭ��Ԫ����__________(��Ԫ�ط���)��
2Cu+SO2���÷�Ӧ�б���ԭ��Ԫ����__________(��Ԫ�ط���)��
(3)��Ӧ(2)�в�����SO2β������NaOH��Һ���գ�����1L 1mol/L��NaOH��Һ���ձ�״����22.4L SO2����Ӧ�����ӷ���ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ͭ������Ļ�ѧʽΪCux(OH)y(CO3)z(CuΪ��2��)��ȡ����Ʒ22.2 g����ּ��Ⱥõ���ɫ��������ͭ16.0 g��������ʵĻ�ѧʽΪ
A.Cu2(OH)4CO3B.Cu3(OH)4CO3
C.Cu2(OH)2CO3D.Cu4(OH)2(CO3)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
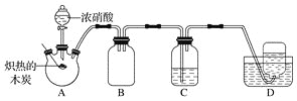
����Ŀ��ij��ѧС�������ͼ��ʾװ�ã���Ũ������ľ̿�ķ�Ӧ����̽��(��֪��4HNO3![]() 4NO2��+O2��+2H2O)��
4NO2��+O2��+2H2O)��
��ش��������⣺
��1�����װ�������Ժ�ȼ�ճ��е�ľ̿�ھƾ����ϼ���������״̬������������ƿ�У����ɵ��������ɫΪ__������������ķ�Ӧ�Ļ�ѧ����ʽ��__��
��2��װ��C��ʢ������Ba(OH)2��Һ�����ȵ�ľ̿��Ũ���ᷴӦ��ɹ۲쵽C�г��ְ�ɫ�������ð�ɫ����Ϊ__(�ѧʽ)��
��3��װ��B��������__��
��4��װ��D���ռ�������ɫ���壬��ͬѧ��Ϊ��NO������ͬѧ��Ϊ��O2��
�����жԸ�����ļ��鷽�����ʵ���__(����ĸ)��
A.���ڹ۲�װ��D�м���ƿ���������ɫ�仯
B.��ʪ�����ɫʯ����ֽ���뼯��ƿ�ڣ��۲���ɫʯ����ֽ�Ƿ���
C.�������ǵ�ľ�����뼯��ƿ�У��۲�ľ���Ƿ�ȼ
�����D�м���ƿ���ռ�������ɫ����������������������Դ��__��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com