
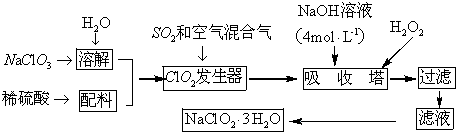
���� �������̿�֪�������ƣ�NaClO3�������������±���������ԭ�ɶ������ȣ�ClO2�ڶ�������Ϳ�����������в��ֽⱬը�����������м�������������Һ�������ⷢ��������ԭ��Ӧ����NaClO2��NaClO2���ܽ�����¶����߶�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ�����NaClO2•3H2O��
��1��ʵ�����ö�������ԭNaClO3����ClO2�����ݵ���غ��Ԫ���غ���д���ӷ���ʽ��
��2������Ϣ�ڿ�֪����ClO2�ֽⱬը��һ����ϡ����������ϡ�ͣ��Է�ֹ��ը��
��3��������Ŀ��Ϣ������������ԭ��Ӧ�����жϷ�Ӧ����������������Ϣ��֪��������������NaClO2��һ����ClO2��NaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض����������������������ݴ���д����ʽ���¶ȹ��ߣ�H2O2���ֽ⣬�ж�NaOH�Ƿ���������÷�̪��
��4������Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ������Ũ������ȴ�ᾧ������ͨ�����˵õ��־��壻
��5���õ��Ĵ־��徭���ؽᾧ�ɵõ����ȸ��ߵľ��壮
��� �⣺��1��ʵ�����ö�������ԭNaClO3����ClO2����Ӧ�����ӷ���ʽΪSO2+2ClO3-=2ClO2+SO42-���ʴ�Ϊ��SO2+2ClO3-=2ClO2+SO42-��
��2������Ϣ�ڿ�֪����ClO2�ֽⱬը���������й������������Ӧ��ϡ��ClO2���Է�ֹ��ը���ʴ�Ϊ��b��
��3������������Ϣ��֪��������������NaClO2������һ����ClO2��NaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض�����������������������Ӧ����ʽΪ2NaOH+2ClO2+H2O2=2 NaClO2+2H2O+O2��H2O2���ȶ����¶ȹ��ߣ�H2O2���ֽ⣬���������¶Ȳ��ܳ���20�棬��Ŀ���Ƿ�ֹH2O2�ֽ⣬��������Ӧά��NaOH�Թ������ж�NaOH�Ƿ���������÷�̪��
�ʴ�Ϊ��2OH-+2ClO2+H2O2�T2ClO2-+O2��+2H2O����ֹH2O2���ȷֽ⣬������NaClO2•3H2O����������̪��
��4������Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ������Ũ������ȴ�ᾧ������ͨ�����˵õ��־��壬�ʴ�Ϊ��b��
��5���õ��Ĵ־��徭���ؽᾧ�ɵõ����ȸ��ߵľ��壮
�ʴ�Ϊ���ؽᾧ��
���� �������������Ʊ�Ϊ�������Թ������ⷨ�Ʊ���������Ϊ���ߣ�����ѧ���Ķ���Ŀ��ȡ��Ϣ����������Ũ�ȸ�������⡢��������ԭ��Ӧ���֪ʶ�����á��й�ʵ������ͼ�ʵ��������������Լ������龳���ۺ�����֪ʶ����������������Ŀ��һ�����Ѷȣ�


 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �Լ״�Ϊԭ�ϣ���KOHΪ�������Һ�Ŀɳ��ĸ�Чȼ�ϵ�أ���绯ѧ������ͼ��
�Լ״�Ϊԭ�ϣ���KOHΪ�������Һ�Ŀɳ��ĸ�Чȼ�ϵ�أ���绯ѧ������ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | �ⶨNaClO��Һ��CH3COONa��Һ��pH | �Ƚ�HClO��CH3COOH������ǿ�� |
| B | ��Mg��OH��2��Һ�еμ�����0.1mol/LFeCl3��Һ | �Ƚ�Mg��OH��2��Fe��OH��3���ܽ�� |
| C | ��������ȫ��ͬ�ҳ���NO2���ܱ���ƿ���ֱ��������ˮ����ˮ�� | ̽���¶ȶԻ�ѧƽ��״̬��Ӱ�� |
| D | ����ֻͬ���ͬŨ�ȵ�K2Cr2O7��Һ�У��ֱ����1mLͬŨ�ȵ�H2SO4��NaOH��Һ | ̽��Ũ�ȶԸû�ѧƽ��״̬��Ӱ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
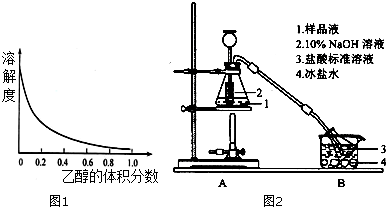
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
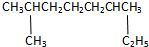 ��ϵͳ�����ǣ�2��6-��������
��ϵͳ�����ǣ�2��6-��������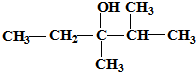 ����ʽ��2��3-����-3-�촼
����ʽ��2��3-����-3-�촼 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϵͳ�������������� 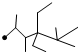 �������� 2��3��5��5-�ļ�-4��4-���һ����� �������� 2��3��5��5-�ļ�-4��4-���һ����� | |
| B�� | �����ʵ����ı��ͱ�������ȫȼ������������������� | |
| C�� | ����ױ���Ϊͬϵ�����ʹKMnO4������Һ��ɫ | |
| D�� | �ṹƬ��Ϊ 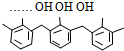 ���ĸ߾���䵥���Ǽ�ȩ�ͱ��� ���ĸ߾���䵥���Ǽ�ȩ�ͱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 6.02��1023 ���ǰ���ӵ����� | |
| B�� | 1 Ħ����������2NA����ԭ�� | |
| C�� | 1 Ħ���κ����ʶ�����6.02��1023 ������ | |
| D�� | 1 Ħ��ˮ����18��6.02��1023 ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com