
����Ŀ��Mn2O3��һ����Ҫ�Ĺ�ҵԭ�ϣ��ڵ�ź��л��ϳɵ�����Ӧ�ù㷺��ijѧϰС����ʵ������CH4��ԭMnO2�Ʊ�Mn2O3�����ⶨ��Ʒ���ȡ���ش��������⣺
��.�Ʊ�(ʵ��װ����ͼ��ʾ)

��֪��Al4C3+12H2O==4Al(OH)3+3CH4����
(1)����a������Ϊ______________________
(2)��ϡ�������ˮ���ŵ���________________________________________
(3)���Ӻ�ʵ��װ�ã�����װ�õ������Բ�������Ӧ�Լ�������ƿ�еμ�ϡ����֮���ڵ�ȼ�ƾ���֮ǰӦ���еIJ�����__________________________________
(4)����b��ͬʱ���������ܲ������ѭ�������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ______________
(5)ָ������ʵ��װ����һ�����Ե�ȱ��____________________________
��.�ⶨ��Ʒ��Mn2O3�Ĵ���(ֻ���Dz�Ʒ�л�������δ���뷴Ӧ��MnO2)
��.ʵ�������ȡ����b�����ù���7.19g���������������ữ��KI��Һ��ʹ��������Ԫ��ȫ��ת��ΪMn2+��
��.��������Һϡ����500mL��
��.ȡ25.00mLϡ�ͺ����Һ���μӼ��ε�����Һ����0.200molL-1��Na2S2O3����Һ�ζ����ﵽ�ζ��յ�ʱ������25.00mLNa2S2O3����Һ��(��֪��I2+2 Na2S2O3==Na2S4O6+2NaI)
��1�����袡�з�����Ӧ�����ӷ���ʽΪ________________________________________
��2���ζ��յ�ı�־��__________________________________________________
��3����ȡ������Mn2O3����������Ϊ___________________(��������ȷ��0.1��)
��4�����в���ʹ�ⶨ���ƫ�ߵ���______________
A.�ζ�ǰƽ�ӣ��ζ��յ㸩�Ӷ���
B.ʢ��Na2S2O3����Һ�ĵζ���δ�ñ�Һ��ϴ
C.�����ữ��KI��Һ�ڿ����о���ʱ�����
D.�ζ�ǰ�������ζ���ζ��ܼ�����������
���𰸡����θ���� �ܽ�Al(OH)3���Al4C3�������� ��װ�ó��ڴ��ռ������鴿 CH4+ 8MnO2 ![]() 4Mn2O3+CO2��+2H2O ȱ�ټ����β������װ�� Mn2O3+2I- +6H+==2Mn2++ I2+ 3H2O MnO2+2I- + 4H+==Mn2++ I2+ 2H2O ���һ��Na2S2O3��Һ���룬��Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ 87.9�� AD
4Mn2O3+CO2��+2H2O ȱ�ټ����β������װ�� Mn2O3+2I- +6H+==2Mn2++ I2+ 3H2O MnO2+2I- + 4H+==Mn2++ I2+ 2H2O ���һ��Na2S2O3��Һ���룬��Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ 87.9�� AD
��������
��.��1�����������Ĺ��켰��;����
��2��ϡ��������ܽ�Al(OH)3��
��3�����鲻����ȼʱ������ը��
��4��������֪��Ϣ��Ԫ���غ���д��ѧ����ʽ��
��5��װ���й����ļ����账����
��. ��1����������Ԫ��ȫ��ת��ΪMn2+������������ԭ��Ӧ��������
��2��I2�����ۻ��������Ӧ���ɵ�I2�����Na2S2O3��Һ��Ӧ����I-���ݴ˷����õ�����ָʾ��ʱ�ﵽ�յ������
��3�����ݹ�ϵʽI2![]() 2 Na2S2O3�ó�I2�����ʵ�������Mn2O3�����ʵ���Ϊx mol��MnO2�����ʵ���Ϊy mol����ϲ��袡�ķ���ʽ����Ԫ���غ�ԭ�����ó����ۣ�
2 Na2S2O3�ó�I2�����ʵ�������Mn2O3�����ʵ���Ϊx mol��MnO2�����ʵ���Ϊy mol����ϲ��袡�ķ���ʽ����Ԫ���غ�ԭ�����ó����ۣ�
��4����Ϲ�ʽc(��) = ![]() �������ݲ��������Բⶨ�����Ӱ���������
�������ݲ��������Բⶨ�����Ӱ���������
��.��1��ͼ��aΪ���θ���ܣ�
�ʴ�Ϊ�����θ���ܣ�
��2�����ݸ���������֪���Ʊ�����Ļ�ѧ����ʽΪ��Al4C3+12H2O==4Al(OH)3+3CH4����ϡ��������ܽ�Al(OH)3�����Al4C3�������ʣ�
�ʴ�Ϊ���ܽ�Al(OH)3���Al4C3�������ʣ�
��3������ȡ�ļ���ɷֲ�����ȼ������ը�������ڵ�ȼ�ƾ���֮ǰӦ���еIJ�������װ�ó��ڴ��ռ������鴿��
�ʴ�Ϊ����װ�ó��ڴ��ռ������鴿��
��4����CH4�ɻ�ԭMnO2����Mn2O3��CԪ������CO2������Ԫ���غ㲢���������ԭ��Ӧ�Ĺ��ɿ�֪�����ܲ������ѭ��������ΪCO2��H2O���仯ѧ����ʽΪ��CH4+ 8MnO2 ![]() 4Mn2O3+CO2��+2H2O��
4Mn2O3+CO2��+2H2O��
�ʴ�Ϊ��CH4+ 8MnO2 ![]() 4Mn2O3+CO2��+2H2O��
4Mn2O3+CO2��+2H2O��
��5����װ����ȱ�ټ����β������װ�ã�
�ʴ�Ϊ��ȱ�ټ����β������װ�ã�
��.��1����������Mn2O3��MnO2��I-���л�ԭ�ԣ�����巴Ӧ���������ӣ�����Ӧ�����ӷ���ʽΪ��Mn2O3+2I- +6H+==2Mn2++ I2+ 3H2O �� MnO2+2I- + 4H+==Mn2++ I2+ 2H2O��
�ʴ�Ϊ��Mn2O3+2I- +6H+==2Mn2++ I2+ 3H2O �� MnO2+2I- + 4H+==Mn2++ I2+ 2H2O��
��2���ζ��յ�ı�־�����һ��Na2S2O3��Һ���룬��Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
�ʴ�Ϊ�����һ��Na2S2O3��Һ���룬��Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��3����Mn2O3�����ʵ���Ϊx mol��MnO2�����ʵ���Ϊy mol����158 g/mol��x + 87��y = 7.19 g �٣���Mn2O3+2I- +6H+==2Mn2++ I2+ 3H2O �� MnO2+2I- + 4H+==Mn2++ I2+ 2H2O�������ɵ�I2�����ʵ���Ϊ��x+y![]() 25
25![]() 10-3 L = 0.005 mol������I2+2 Na2S2O3 =Na2S4O6+2NaI��֪����Ӧ��I2�����ʵ��� =
10-3 L = 0.005 mol������I2+2 Na2S2O3 =Na2S4O6+2NaI��֪����Ӧ��I2�����ʵ��� = ![]() ��
��![]() = 0.05 mol����x+y = 0.05 mol �ڣ���Ϣٺ͢�ʽ��ã�x = 0.04��y = 0.01������Mn2O3����������Ϊ
= 0.05 mol����x+y = 0.05 mol �ڣ���Ϣٺ͢�ʽ��ã�x = 0.04��y = 0.01������Mn2O3����������Ϊ![]()
![]() 87.9%��
87.9%��
�ʴ�Ϊ��87.9%��
��4��A. �ζ�ǰƽ�ӣ��ζ��յ㸩�Ӷ��������Һ���������ƫС��c(��) = ![]() ������Һ��I2�����ʵ���ƫС��ͨ������ó�����ʹMn2O3�Ĵ���ƫ�ߣ�A����ȷ��
������Һ��I2�����ʵ���ƫС��ͨ������ó�����ʹMn2O3�Ĵ���ƫ�ߣ�A����ȷ��
B. ʢ��Na2S2O3����Һ�ĵζ���δ�ñ�Һ��ϴ����ҺŨ��ƫ�ͣ����ĵı�Һ���ƫ��I2�������ʵ���ƫ��ͨ������ó�����ʹMn2O3�Ĵ���ƫ�ͣ�B�����
C.�����ữ��KI��Һ�ڿ����о���ʱ���������Һ�е�I-���ֻᱻ������I2�������ĵ�Na2S2O3��Һ���ƫ��ͨ������ó�����ʹMn2O3�Ĵ���ƫ�ͣ�C�����
D.�ζ�ǰ�������ζ���ζ��ܼ����������ݣ����Һ���������ƫС������c(��) = ![]() ������Һ��I2�����ʵ���ƫС��ͨ������ó�����ʹMn2O3�Ĵ���ƫ�ߣ�D����ȷ��
������Һ��I2�����ʵ���ƫС��ͨ������ó�����ʹMn2O3�Ĵ���ƫ�ߣ�D����ȷ��
��ѡAD��


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ��������ϸ������Ʒ����Ҫ�м��壬���Ʊ��漰�ķ�Ӧ���£�
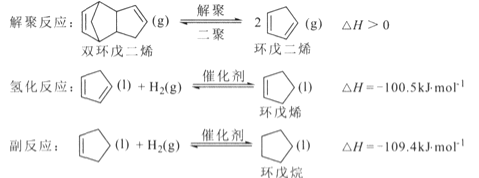
�ش��������⣺
(l)��Ӧ![]() �ġ�H= _________ kJ/mol ��
�ġ�H= _________ kJ/mol ��
(2)��۷�Ӧ�ڸ��������н��С�
�������������䣬���������˫�����ϩƽ��ת���ʵ������� ____ �����ţ�.
A.�����¶� B�������¶� C������ѹǿ D����Сѹǿ
��ʵ�������г�ͨ��ˮ�����Խ���˫�����ϩ�ķе㡣ij�¶��£�ͨ����ѹΪl00kPa��˫�����ϩ��ˮ�������ﵽƽ�����ѹΪ160kPa��˫�����ϩ��ת����Ϊ8 0%���� pH2O=___kpa��ƽ�ⳣ��Kp=______kPa (KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��)
(3) һ�������£��������ϩ�����л��ܼ��н����⻯��Ӧ����Ӧ�����б�������ѹ�����䣬��û���ϩ�ͻ�����IJ��ʣ��Ի����ϩΪԭ�ϼƣ���ʱ��仯����ͼ��ʾ��

�ٽ������ϩ�����л��ܼ��пɼ��ٶ��۷�Ӧ�ķ�����ԭ����____��
����ѵķ�Ӧʱ��Ϊ__h����ܽϴ����__����⻯��Ӧ������Ӧ������
(4)��֪�⻯��Ӧƽ�ⳣ��Ϊ1.6 �� 1012������Ӧ��ƽ�ⳣ��Ϊ2.0��10l2���ں��º����£������ϩ�����������ʵ���֮��Ϊ1��1���з�Ӧ�������ϩ�ĺ�����ʱ��仯������____�������ǻ����ϩ�Ķ��۷�Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л����������ȷ����
A.�����ع��������з���ɵõ����ͻ����B.�����ʲ��ܷ���������Ӧ
C.���ۺ���ά�ز���Ϊͬ���칹��D.���ñ�����ˮ�����������¿��Ƶ��屽
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������25 �������½�pH��12�İ�ˮϡ�����У����й�ϵʽ��ȷ����________��
A����ʹ��Һ��c(NH4+)��c(OH��)����
B����Һ��c(H��)��c(OH��)����
C����ʹ��Һ��![]() ��ֵ����
��ֵ����
D���˹�����Kw����
����25 �������½�pH��12�İ�ˮϡ��100������Һ��pHΪ________(�����)��
A��10 B��11 C��10��12 D��11��13
(2)25 ��ʱ����0.1 mol��L��1�İ�ˮ�м��������Ȼ�粒��壬�������ܽ�����ҺpH��С����Ҫԭ����________(�����)��
�ٰ�ˮ���Ȼ�立�����ѧ��Ӧ
���Ȼ����Һˮ�������ԣ�������c(H��)
���Ȼ������ˮ���������������ӣ�������һˮ�ϰ��ĵ��룬ʹc(OH��)��С
(3)�����£������0.2 mol NH4Cl��0.1 mol NaOHȫ������ˮ���γɻ����Һ(��������ʧ)��
��________��________�������ӵ����ʵ���֮�͵���0.2 mol��
��________��________�������ӵ����ʵ���֮�ͱ�OH����0.1 mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ļ�����ϳɡ�Ӧ���Լ��Ի�����Ӱ��һֱ�ǿ�ѧ���о����ȵ㡣
(1)������Ҫ��NH3��CO2Ϊԭ�Ͻ��кϳɡ���Ҫͨ������������Ӧ����:
��Ӧ1��2NH3(l)+CO2(g)![]() H2NCOONH4(l)����H1= -117.2 kJ��mol-1
H2NCOONH4(l)����H1= -117.2 kJ��mol-1
��Ӧ2��H2NCOONH4(l)![]() H2O(l)+CO(NH2)2(l)����H2=+21.7 kJ��mol-1
H2O(l)+CO(NH2)2(l)����H2=+21.7 kJ��mol-1
��ش�
CO(NH2)2 (l)+H2O(l)![]() 2NH3(l)+CO2(g) ����H3=____ kJ��mol-1;�÷�Ӧ�ܷ�������Ҫԭ����_________
2NH3(l)+CO2(g) ����H3=____ kJ��mol-1;�÷�Ӧ�ܷ�������Ҫԭ����_________
(2)��̿����ԭSO2����S2����ѧ����ʽΪ��2C(s)+2SO2(g)S2(g)+2CO2(g)���ں��������У�1 mol/LSO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ��ʾ��

����700�淢���÷�Ӧ����3���Ӵﵽƽ�⣬����0��3����v(S2)=_______molL-1min-1�����¶��µ�ƽ�ⳣ��Ϊ_________
�����÷�Ӧ����ʼ�¶�Ϊ700��ĺ��ݾ��������н��У��ﵽƽ��ʱSO2��ת����_________90%(��������������������=��)
������˵��һ����˵���÷�Ӧ�ﵽƽ��״̬����____
A.��̿���������ٱ仯ʱ
B.CO2��SO2��Ũ�����ʱ
C.SO2������������CO2����������֮��Ϊ1:1
D.��������ѹǿ���ٱ仯ʱ
(3)��֪25��ʱ��H2SO3�ĵ��볣��Ka1=1.3��10-2��Ka2=6.2��10-8
�ٹ�ҵ�Ͽ���Na2SO3��Һ���շ�����SO2��25��ʱ��1mo/L��Na2SO3��Һ����SO2������ҺpH=7ʱ����Һ�и�����Ũ�ȵĴ�С��ϵΪ___________________
�ڹ�ҵ��Ҳ���ð�ˮ������SO2��д����ˮ���չ���SO2�Ļ�ѧ��Ӧ����ʽ______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ͬ��ͬѹ�£�a g�������2a g��������ռ���֮��Ϊ1��2�����ҵ��ܶ�֮��Ϊ______��
��2����������������������̼��ɵĻ��������ͬ�¡�ͬѹ����Ц����N2O�����ܶ���ͬ����û�������ж�������������������̼������ȿ���Ϊ_______������ĸ����
A��42:20:13 B��22:1:14 C��13:8:13 D��21:10:12
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ij��ȤС������ͼװ����ͨ����н�������ʵ�飺
ʵ�� | ���� |
| �Թ��п�ʼ������,����С��������,Խ��Խ����,Һ���Ϸ�����dz����ɫ����,��Һ����ɫ. |
�Թ��о��ҷ�Ӧ,Ѹ�����ɴ�������ɫ����, ��Һ����ɫ;֮������ɫ��Һ�г���ͨ��N2, ��Һ��Ϊ��ɫ. |
����˵����ȷ����
A. �Թ�����dz����ɫ����ΪNO2,�����ỹԭ����
B. �������� Cu ��ȫ�ܽ�ʱ,�������ĵ�HNO3����
C. ����Fe֮���ظ�ʵ��,��Ȼ���Թ����з�Ӧ������
D. �Թ����з�Ӧ����Һ��ɫ���Թ����еIJ�ͬ,����������NO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������и������ʣ�
�ٺ��ס����� ��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
��������ͬλ�ص���______________������ͬ�����������___________������ͬ��Ԫ�صIJ�ͬ��������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������A��B��C��D����Ԫ�أ���֪AԪ���ǵؿ��к�������Ԫ�أ�BԪ��Ϊ����Ԫ�أ�����ԭ�Ӻ���K��L���ϵ�����֮�͵���M��N�������֮�ͣ�CԪ���ǵ������ڵ�һ��������С��Ԫ�أ�DԪ���ڵ��������е縺�����
��1�����ƶ�A��B��C��D����Ԫ�صķ��ţ�A________��B________��C________��D________��
��2��д��AԪ��ԭ�ӵĺ�������Ų�ʽ��____________��д��BԪ��ԭ�Ӻ�������Ų��ļ۵��ӹ��ͣ�__________���õ����Ų�ͼ��ʾCԭ�ӵĺ�������Ų������_______________��
��3���Ƚ�����Ԫ�صĵ�һ�����ܺ͵縺�ԵĴ�С����һ������____________���縺��____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com