
������ĺ���������ҽҩ�������ȷ���������Ҫ����;��
��1��)�ؾ�ʯ��BaS04)����θ������Ӱ����
��֪�������£�Ksp(BaSO4)=1.1��10-10����BaSO4����Һ�м������ᣬ����Һ��pH=2ʱ�� ��Һ�� c(Ba2+)= ��
��2����������茶���[(NH4)2Fe(SO4)2��6H20]������������
�ټ��龧���к���NH4+�ķ���Ϊ_______��
�ڵ����ʵ���Ũ�ȵ�����ϡ��Һ��
a��(NH4)2Fe(SO4)2 b��NH4HSO4 c��(NH4)2SO4 d��(NH4)2SO3
����C(NH4+)�ɴ�С��˳��Ϊ ����ѡ����ĸ����
��3����������أ�K2S2O8)����ǿ��������Na2S2O3������ԭ����
��K2S2O8��Һ������MnSO4��Һ���,�ڴ��������£����Թ۲쵽��Һ��Ϊ��ɫ�� �÷�Ӧ�����ӷ���ʽΪ
���ò����缫�����H2SO4��K2SO4�Ļ����Һ�����Ʊ�K2S2O8���������ĵ缫��ӦʽΪ_____ __������������������Һ��pH��______ (���������С�����䡱)
�۲�Ʒ��K2S2O8�ĺ������õ������ⶨ����������Ϊ����ȡ0.3g��Ʒ�ڵ���ƿ�У���50 mLˮ�ܽ⣻����4.000 g KI����(�Թ���������ʹ���ַ�Ӧ����������������Һ�ữ����______Ϊָʾ������0.1000 mol��L-1 Na2S2O3��Һ�ζ����յ㣨��֪��I2��2S2O32��=2I����S4O62�������ظ�2�Σ����ƽ�����ı�Һ21.00mL���ò�Ʒ��K2S2O8����������Ϊ(���ʲ��μӷ�Ӧ) (��ʽ�����㣩��
��1��2.2��10��8 mol/L ��2�֣�
��2���� ȡ�������壬��ˮ�ܽ⣬�ټ�������NaOHŨ��Һ���ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬����NH4+��������������Ҳ���֣� ��2�֣�
�� a��c��d��b ��2�֣�д��a c d b ��b��d��c��aҲ�ɸ��֣���д��b d c a�����֣�
��3���� 5S2O82��+2Mn2++8H2O����10SO42��+2MnO4��+16H+ ��2�֣�
�� 2SO42�� ��2e��=S2O82������2HSO4�� ��2e�� = S2O82��+2H+�� ��2�֣�
���� ��1�֣�
�� ���� ��1�֣�
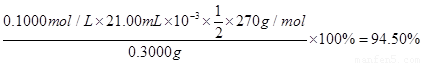
��3�֣���ʽ2�֣����1�֡���ʽ�в�����λ���۷֣�д��94.5%��۷֡���
��������
�����������1��pH=2ʱ��c(SO42-)=0.5��10-2mol/L��Ksp(BaSO4)=c(Ba2+)��c(SO42-)=1.1��10-10����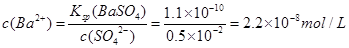 ��
��
��2����NH4+�ļ���ͨ���ǼӼʹ��ת��Ϊ��������ʪ���ʯ����ֽ���鰱����
��NH4+�����������ӣ���ˮ�п�ˮ��ʹ��Һ�����ԡ�a��NH4+��Fe2+��ˮ������ƣ�c��NH4+����ˮ�⣬d��NH4+��SO32-ˮ����ٽ������c(NH4+)�ɴ�СΪa>c>d����a��c��d�����ж���2��笠����ӣ���b������ֻ��1��笠����ӣ�ˮ��Ͼ������ģ����b�е�c(NH4+)��С��������˳��Ϊa��c��d��b��
��3������Һ����ɫ˵���и���������ɣ��������������ԭΪ�������
�ڵ��ʱ��������������ʧ���ӣ��ʵ缫��ӦʽΪ2SO42�� ��2e��=S2O82�������������ӵõ��ӣ�������ӦΪ2H++2e��=H2���������Ӽ�����Һ���Լ�����pH���ߡ�
�۸������������ζ�ԭ����Na2S2O3��Һ�����ζ��ⵥ�ʣ��������۱���������õ�����ָʾ����
�ζ�ʱ�����ķ�Ӧ�У�S2O82��+2I��=2SO42��+I2��I2��2S2O32��=2I����S4O62��������S2O82����2S2O32������˲�Ʒ��K2S2O8������Ϊ0.1000��21��10-3��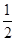 ��270=0.2835g����Ʒ��K2S2O8����������Ϊ
��270=0.2835g����Ʒ��K2S2O8����������Ϊ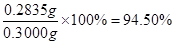 ��
��
���㣺�����ܶȻ��ļ��㣬�����ˮ�⣬���Ӽ��飬����ԭ�����к͵ζ�ԭ����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ �� �� �� | Ԥ������ʵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| n(SO32-) |
| n(HSO3-) |
|
91��9 | 1��1 | 9��91 | ||
| ������pH | 8.2 | 7.2 | 6.2 |
| n(SO32-) |
| n(HSO3-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com