
����Ŀ���±���A��B��C��D��E�����л�����й���Ϣ��
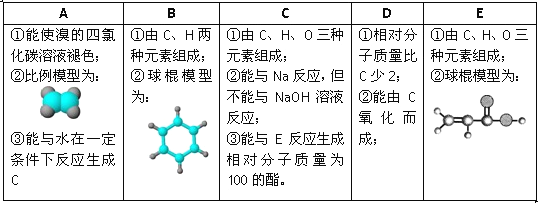
���ݱ�����Ϣ�ش��������⣺
(1)A��E�У�����������_______(����ĸ)��д��A����ˮ��Ӧ�Ļ�ѧ����ʽ_____________��
(2)A�����������ӳɷ�Ӧ�����ɷ���F����F�ڷ�����ɺͽṹ�����Ƶ��л�����һ����(�׳ơ�ͬϵ�)�����Ǿ�����ͨʽ_______����n=________ʱ�������л��↑ʼ����ͬ���칹�塣
(3)B���������_____________(�����)��
����ɫ��ζҺ�� ���ж� �۲�����ˮ ���ܶȱ�ˮ��
���κ������²���������Ӧ��ʹ���Ը��������Һ����ˮ����ɫ
(4)д����Ũ���������£�B��Ũ���ᷴӦ�Ļ�ѧ����ʽ��_________________��
(5)C��E��Ӧ��������Է�������Ϊ100�������÷�Ӧ����Ϊ_____________���仯ѧ����ʽΪ��______________________��
���𰸡� A B CH2=CH2 + Br2��CH2BrCH2Br CnH2n+2 4 �ڢ� ![]() ������Ӧ(��ȡ����Ӧ) CH2��CH��COOH �� C2H5OH
������Ӧ(��ȡ����Ӧ) CH2��CH��COOH �� C2H5OH ![]() CH2��CH��COOC2H5 �� H2O(2��)
CH2��CH��COOC2H5 �� H2O(2��)
��������A��ʹ���CCl4��Һ��ɫ˵������̼̼˫������������ϱ���ģ��֪��AΪ����ϩ��A����ˮ��һ�������·�Ӧ����C����CΪ�Ҵ�������B��C��H����Ԫ����ɼ������ģ�Ϳ�֪��BΪ����D����Է���������C��2������C�������ɣ�����DΪ��ȩ����E�����Ԫ�ؼ����ģ�Ϳ�֪��EΪCH2=CH-COOH��
��1��������������5���л�����ֻ����ϩ�ͱ�����������A��E�У�������������A��B����ϩ����ˮ�����ӳɷ�Ӧ����ѧ����ʽΪ��CH2=CH2+Br2��CH2BrCH2Br��
��2��AΪ��ϩ�����������ӳɷ�Ӧ������F��FΪ���飬��F�ڷ�����ɺͽṹ�����Ƶ��л�������������ͨʽΪ��CnH2n+2����n=4ʱ�����Ӷ��鿪ʼ����ͬ���칹�塣
��3��BΪ���������±�����ɫ����������ζ��Һ�����ܶȱ�ˮС��������ˮ���ж����������к��в����ͼ���һ�������¿��������������ӳɷ�Ӧ������̼̼˫��������������ʹ���Ը��������Һ����ˮ����ɫ�����ڢ���ȷ��
��4����Ũ���������£�����Ũ���ᷢ��������Ӧ��ȡ����Ӧ����������������ѧ����ʽΪ��![]() ��
��
��5��CΪCH3CH2OH����Է�������Ϊ4��EΪCH2=CH-COOH����Է�������Ϊ72�����߷���������Ӧ����ȡ����Ӧ��������Է�������Ϊ100�������仯ѧ����ʽΪ��CH2=CH-COOH��C2H5OH ![]() CH2=CH-COOC2H5��H2O��
CH2=CH-COOC2H5��H2O��


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ��Ӧ�����ӷ���ʽ��ȷ����
A������ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2��
B��ͭ��ϡ���ᷴӦ��Cu+2H+=Cu2+ +H2��
C��ʵ������ȡCO2���壺CO32-+2H+=CO2��+H2O
D����������ͭ��Һ��Ӧ��Fe+Cu2+=Fe2++Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й�������������У���ȷ���ǣ�������
A.Ũ���������ˮ�ԣ������ʹ����̿��
B.Ũ������ǿ�����ԣ�����������������������
C.Ũ������һ�ָ�������ܹ����ﰱ��������������
D.ϡ��Ũ����ʱ��Ӧ��ˮ���������������뵽Ũ�����У����ò��������Ͻ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��̬��A �� H2������ܶ�Ϊ14�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ���Ըû�����Ϊԭ���ϳɻ�����G��E��I���������£�
��֪����.�����廯����FΪC��H��O���������Է�������Ϊ166�����ϵ�һ�ȴ�����һ�֣�1 mol F������NaHCO3��Һ��Ӧ������2 mol CO2��F������B��Ӧ����G��
��HΪ��Ԫ�����������ܶ�����ɱ�״��Ϊ2.77 g/L��H������D��Ӧ����I��

(1)A�ĵ���ʽΪ____________________��E�Ľṹ��ʽ____________________________________��
(2)G�ķ���ʽΪ______________����Ӧ�ݵķ�Ӧ����Ϊ________________��
(3)д�����л�ѧ����ʽ��
��__________________________________________________________________��
��_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ѡ��һ���Լ���ȥ���и������е����ʣ�������Ϊ���ʣ�������ѡ�Լ��Ļ�ѧʽ���ں����ϣ���д�����ӷ���ʽ��
��1��BaCl2(HCl)�Լ� �����ӷ���ʽΪ ��
��2��CO(CO2)�Լ� �����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Q����W�ĺϳ�·�����£�
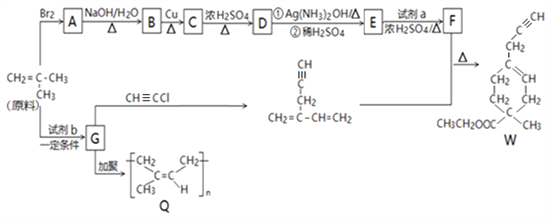
��֪��
��
��
(R��R����������ԭ�ӡ������������)
��1����ϵͳ�������У�A��������_________���ϳ�A�ķ�Ӧ������__________��
��2��B�����������ŵ�������____________��
��3���Լ�b�Ľṹʽ��____________��
��4������˵���У���ȷ��____________��
�� �Լ�aΪ�״�
�� C��D��D��E��Ӧ����ǰ��
�� �γ�![]() �ĵ����к�G
�ĵ����к�G
�� ��Q���ױ�Br2��ʴ
��5��C��D�Ļ�ѧ����ʽ��___________��
��6���Լ�b�뱽���γɸ߷��ӻ�����Ļ�ѧ����ʽ��_____________��
��7��F�Ľṹ��ʽ��_____________��
��8����CH2=CH-CH=CH2��HOCH2CH=CHCHOΪԭ���Ʊ�![]() ��
��
��ϳ�·��ͼ�ǣ�____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������£���һԪ��HA����Һ��KOH��Һ�������ϣ����Ի�Ϻ���Һ������仯����ʵ���������±���
ʵ����� | ��ʼŨ�ȣ���mol��L��1�� | ��Ӧ����Һ��pH | |
c��HA�� | c��KOH�� | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
��ش�
��1��HA��Һ��KOH��Һ��Ӧ�����ӷ���ʽΪ________��
��2��ʵ������Ӧ�����Һ����ˮ�������c��OH������________mol��L��1��x________0.2mol��L��1����������������������������
��3�����й���ʵ������Ӧ�����Һ˵������ȷ����________������ĸ����
a����Һ��ֻ����������ƽ��
b����Һ�У�c��A������c��HA����0.1mol��L��1
c����Һ�У�c��K������c��A������c��OH������c��H����
������֪2H2��g����O2��g����2H2O��1�� ��H����572kJ��mol��1��ij����ȼ�ϵ�������ɶ��ʯī��Ϊ�缫��KOH��ҺΪ�������Һ��
��4��д���õ�ع���ʱ�����ĵ缫��Ӧʽ________��
��5����������ȼ�ϵ��ÿ�ͷ�228.8kJ����ʱ��������1molҺ̬ˮ����õ�ص�����ת����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ� ��
A. ��������ˮ��Ӧ��Na+ H2O=Na++ H2
B. �Ȼ����Һ�����Ե�ԭ��NH4++H2O=NH3��H2O+H+
C. AICl3��Һ�м���������ˮ��Al3++4NH3-H2O= AlO2-+4NH4+2H2O
D. �Ȼ�����Һ�еμ�������������Һ��2Fe3++H2S= 2Fe2++S+2H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС�������һ��ʵ����̽��Ԫ�������ɡ�
��ͬѧ����Ԫ�طǽ��������Ӧ��ۺ�����֮��Ĺ�ϵ���������ͼ1װ����һ�������ͬ����Ԫ��C��Si�ķǽ�����ǿ���Ƚϵ�ʵ���о���
��ͬѧ�������ͼ2װ������֤±��Ԫ�����ʵĵݱ���ɣ�ͼ2��A��B��C�����ֱ���մ��NaBr��Һ������ʪ��ĵ���KI��ֽ��ʪ��ĺ�ֽ��
��֪������Ũ�������������ܷ�Ӧ����������

��1������������������ѡ����ͬѧ��Ƶ�ʵ�����õ����ʣ�ͼ1���Լ�A��BΪ������ţ�____��
��ϡ������Һ�� ��Ũ��� ��̼���Ʒ�ĩ����Na2SO3��Һ
д��ͼ1��ƿ�з�����Ӧ�����ӷ���ʽΪ__________________________________________��
��2����ͬѧ����ʵ��ͼ1�ձ�������Ϊ____________________________________��
��3����ͬѧ����ʵ��ͼ2��B��������Ϊ__________________________________��
��4��д��ͼ2��A��������Ӧ�����ӷ���ʽΪ__________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com