

 BaTiO3+CO2�� ; 6 ; 233/[NA����a��10-7��3]
BaTiO3+CO2�� ; 6 ; 233/[NA����a��10-7��3] BaTiO3+CO2�����ھ�����ÿ��Tiԭ����Χ��6��Oԭ������������ȶ����������6��Oԭ�ӹ��ɵ����������塣������λ��Ϊ6.������һ��������ֻ����һ��BaTiO3�����Ծ�����ܶ�
BaTiO3+CO2�����ھ�����ÿ��Tiԭ����Χ��6��Oԭ������������ȶ����������6��Oԭ�ӹ��ɵ����������塣������λ��Ϊ6.������һ��������ֻ����һ��BaTiO3�����Ծ�����ܶ�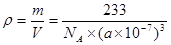 ��
��

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ԭ�Ӱ뾶��Y>Z>W |
| B��XW2�۵�Ϊ��107��C���е�Ϊ12.5��C��˵����̬XW2Ϊ���Ӿ��� |
| C��YW3ˮ��Һ���ɲ����տɵ�YW3���� |
D��ZW2���ӽṹ����ͼ��˵��ZW2�Ǽ��Է��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ����ʾ���Ԫ�ص�ԭ���г�ȥ�������ӵ�ʣ�ಿ�֣���
����ʾ���Ԫ�ص�ԭ���г�ȥ�������ӵ�ʣ�ಿ�֣��� ����ʾ��ԭ�ӣ�С�ڵ㡰
����ʾ��ԭ�ӣ�С�ڵ㡰 ����ʾû���γɹ��ۼ����������ӣ����߱�ʾ���ۼ���
����ʾû���γɹ��ۼ����������ӣ����߱�ʾ���ۼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 4NaCl+O2;2CaO+2Cl2
4NaCl+O2;2CaO+2Cl2 2CaCl2+O2;SiO2+2CCl4
2CaCl2+O2;SiO2+2CCl4 SiCl4+2COCl2;Cr2O3+3CCl4
SiCl4+2COCl2;Cr2O3+3CCl4 2CrCl3+3COCl2��
2CrCl3+3COCl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��1.732)
��1.732)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A��Ԫ�ص縺���ɴ�С��˳��ΪF��O��N |
| B��һ�������Ӻ�3���м���6���Ҽ� |
| C���Ȼ��ƺ��Ȼ�菉����������ӵ���λ����ͬ |
| D����һ�����ܵĴ�СΪBr��Se��As |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com