��2012?������ģ�����᳧�����ջ�����FeS
2������ȡ���ᣬʵ�����������᳧��������Ҫ�ɷ���Fe
2O
3������FeS��SiO
2���Ʊ��̷���
��1��SO
2��O
2��Ӧ��ȡSO
3�ķ�Ӧԭ��Ϊ��2SO
2+O
22SO
3����һ�ܱ�������һ��ʱ���ڴﵽƽ�⣮
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��
��
�ڸ÷�Ӧ�ﵽƽ��״̬�ı�־��
BD
BD
��
A��v��SO
2��=v��SO
3�� B��������ƽ����Է�����������
C����������������� D������ֵ������������
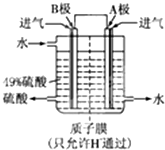
��2��ij���е�λ����ԭ���ԭ������SO
2��O
2���Ʊ����ᣬװ����ͼ���缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���
��B�缫�ĵ缫��ӦʽΪ
SO2-2e-+2H2O�TSO42-+4H+
SO2-2e-+2H2O�TSO42-+4H+
��
����Һ��H
+���ƶ�������
B
B
����
A
A
����
����ܷ�ӦʽΪ
2SO2+O2+2H2O�T2H2SO4
2SO2+O2+2H2O�T2H2SO4
��
��3�������������̷��Ĺ������£�
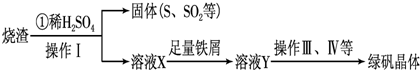
�ⶨ�̷���Ʒ�к�����ʵ�鲽�裺
a����ȡ5.7g��Ʒ���ܽ⣬���250mL��Һ
b����ȡ25mL����Һ����ƿ��
c���������ữ��0.01mol/L KMnO
4��Һ�ζ����յ㣬����KMnO
4��Һ���40mL
������������ش��������⣺
�ٵζ�ʱ������Ӧ�����ӷ���ʽΪ����ɲ���ƽ���ӷ�Ӧ����ʽ����
5
5
Fe
2++
1
1
Mn+
8
8
H+
H+
--
5
5
Fe
3++
1
1
Mn
2++
4
4
H2O
H2O
���������ữ��KMnO
4�ζ��յ�ı�־��
�ζ����һ������KMnO4ʱ��Һ�ʵ���ɫ��������ڲ���ɫ
�ζ����һ������KMnO4ʱ��Һ�ʵ���ɫ��������ڲ���ɫ
��
�ۼ���������Ʒ��FeSO
4?7H
2O����������Ϊ
0.975��97.5%
0.975��97.5%
��






 ��2012?������ģ��A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ�����ַ�Ӧ�е�ˮ����ȥ����
��2012?������ģ��A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ�����ַ�Ӧ�е�ˮ����ȥ����