��2010?������ģ�⣩��̼������һ���ж���;��������ϵƯ������ѧʽ�ɱ�ʾΪaNa
2CO
3?bH
2O
2���ֽ�һ�������Ĺ�̼���Ʒ�ĩ����ˮ���ϡ��Һ���������м�������MnO
2��ĩ����ַ�Ӧ������672mL���壨��״��������Ӧ��Ļ���ᆳ���ˡ�ϴ�Ӻ���Һ��ϴ��Һ��ϲ���ˮ���100mL��ҺA�������������Ϊ10mL��Ũ��Ϊcmol/L��ϡ�����зֱ���ε�����ҺA���ߵα�����ַ�Ӧ���ʵ���������±���ʾ��
| ʵ����� |
�� |
�� |
�� |
| �μ���ҺA�����/mL |
10.0 |
20.0 |
30.0 |
| ������������/mL����״���� |
89.6 |
179.2 |
224 |
��1������ϡ��������ʵ���Ũ�ȣ�
��2��ͨ������ȷ����̼���ƵĻ�ѧʽ��
��3����ҵ�ϳ�����������������������[w����������=
��100%]��������̼���Ʋ�Ʒ�����ӣ�13%������Ϊ�ŵ�Ʒ���ֽ�0.2gij���������Ĺ�̼������Ʒ���������ʲ���������������ԭ��Ӧ������ˮ�����Һ������15.0mL 1mol/L���ᣬ�ټ�������KI��ҡ�Ⱥ����ڰ�������ַ�Ӧ��������������Һ����0.1mol/L Na
2S
2O
3��Һ�ζ�����ɫǡ����ʧʱ��������30.00mL���Լ����жϸ���Ʒ�Ƿ�Ϊ�ŵ�Ʒ������֪��2Na
2S
2O
3+I
2��Na
2S
4O
6+2NaI��




 ��R1��R2��R3����������
��R1��R2��R3����������

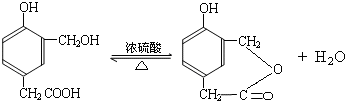

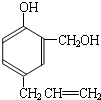

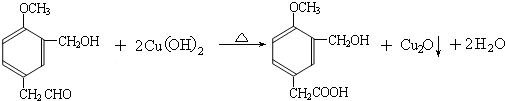

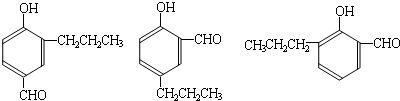

 ���ĺϳ�
���ĺϳ� ·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������
·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������
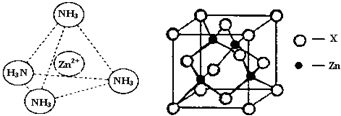
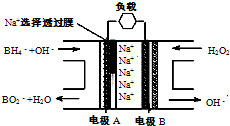 ��2010?������ģ�⣩ֱ��NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ�����������ϲ���Pt/C���������ϲ���MnO2�����йظõ�ص�˵����ȷ����
��2010?������ģ�⣩ֱ��NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ�����������ϲ���Pt/C���������ϲ���MnO2�����йظõ�ص�˵����ȷ����