
��ѡ���⣬10�֣���ij��̬����̼85.7%������14.3%���ڱ�״���µ��ܶ���2.5 g/L��������ʹ���Ը��������Һ����ˮ��ɫ��
(1)��������ʽΪ_______________________��
(2)д�����ĸ���ͬ���칹��Ľṹ��ʽ�� __________________________________��
��44.8 gͭ��140 mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ⣬������NO��NO2��������ڱ�״���µ����Ϊ11.2L��ش�
(1)����£�NO2�����Ϊ L
(2)������������ȫ���ͷź�����Һ�м���V mL a mol/L��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת���ɳ�������ԭ������ҺŨ��Ϊ �������ʽ��
(3)��ʹͭ�����ᷴӦ���ɵ�������O2��H2Oȫ��ת��ΪHNO3������ҪO2 g
26����10�֣���ÿ��1�֣���ÿ��2�֢� (1)C4H8 (2) CH2=CHCH2CH3 CH3CH=CHCH3 CH2=C(CH3)2
��1��1.12 (2)
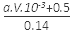 ��3��11.2
�� ��2�֣�
��3��11.2
�� ��2�֣�
���������������֪������ʹ���Ը��������Һ����ˮ��ɫ��˫����C:H=1:2.M=56g�Mmol,��������ʽΪC4H8 , ���ĸ���ͬ���칹��Ľṹ��ʽ: CH2=CHCH2CH3 CH3CH=CHCH3 CH2=C(CH3)2.
Cu+4HNO3Ũ=Cu(NO3)2+NO2��+2H2O 3Cu+8HNO3Ũ=3Cu(NO3)2+2NO��+4H2O
x x 1.5y y
64(x+1.5y)= 44.8 g x+y=0.5mol x= 0.1mol y=0.4mol
NaOH---------HNO3--------0.5 Cu2+
10-3av 0.7mol n(N)= 10-3av +0.5



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||


 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 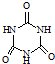 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ��| ������ | �ܶ�/g?cm-3 | �е�/�� | �ܽ��/100gˮ |
| ������ | 0.810 | 118.0 | 9 |
| ������ | 1.049 | 118.1 | �� |
| ���������� | 0.882 | 126.1 | 0.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���л���ѧ������ģ������
1.�л��ϼ����Ϲ�����Һ̬��С�����ϼ�����ѧ��Ӧת��Ϊ����ӻ�߷��Ӷ��̻���
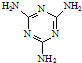
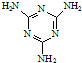
(1)��502������һ�ֿ�ɽ�������Ҫ�ɷ�Ϊ�������ϩ������( )����д����502�������������õĻ�ѧ����ʽ��_____________________________����Ӧ������_____________��
)����д����502�������������õĻ�ѧ����ʽ��_____________________________����Ӧ������_____________��
(2)������( )Ҳ��һ���ϼ�����ҵ���ñ�ϩ���ij������һ�������·�Ӧ���Ƶ������ϼ��������ʵ�������_____________����д����һ��ȡ���̵Ļ�ѧ����ʽ_________________��
)Ҳ��һ���ϼ�����ҵ���ñ�ϩ���ij������һ�������·�Ӧ���Ƶ������ϼ��������ʵ�������_____________����д����һ��ȡ���̵Ļ�ѧ����ʽ_________________��
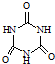
(3)���齺�dz��õ��ϼ�������Ҫ�ɷ�Ϊ������ϩ��(CH3COOCH=CH2)�����ж���ͬ���칹�壬�� ��
�� ��
�� ����֪����
����֪����![]() �ṹ�����ʲ����ȶ����ڡ�������д��3�ֺ���CH=CH���ṹ����״ͬ���칹��Ľṹ��ʽ��______________��______________��______________��
�ṹ�����ʲ����ȶ����ڡ�������д��3�ֺ���CH=CH���ṹ����״ͬ���칹��Ľṹ��ʽ��______________��______________��______________��
(4)��֪����ȩ���Է�����Ӧ��2CH3OH+HCHO![]() CH3OCH2OCH3+H2O������ϩ����������ͨ��ˮ�������ǻ���ȫ���붡ȩ(CH3CH2CH2CHO)������ˮ���õ�����ԭ�ӻ���ǿ���ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ��________________��
CH3OCH2OCH3+H2O������ϩ����������ͨ��ˮ�������ǻ���ȫ���붡ȩ(CH3CH2CH2CHO)������ˮ���õ�����ԭ�ӻ���ǿ���ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ��________________��
�����ʽṹ�����ʡ�ģ������2.����ͼ��Na��Cu��Si��H��C��N��Ԫ�ص��ʵ��۵�ߵ͵�˳������c��d�����Ⱥ͵�������塣

(1)��д����ͼ��d���ʶ�ӦԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽ_____________��
(2)����a��f��b��Ӧ��Ԫ����ԭ�Ӹ�����1��1��1�γɵķ����к�_____________���Ҽ���_____________���м���
(3)a��b��Ԫ���γɵ�10�������Է���X�Ŀռ乹��Ϊ_____________����X����ˮ�����Һ���뵽��dԪ�ظ����ӵ���Һ�������������ɵĺ�dԪ�����ӵĻ�ѧʽΪ________
_______������X��d�ĸ�����֮����_____________����ϡ�
(4)��������Ԫ���е�һ��Ԫ���γɵĺ�����ĽṹΪ��![]() ���ú�����ķ���ʽΪ_____________�����Ҫ˵��������������ˮ��ԭ��__________________________��
���ú�����ķ���ʽΪ_____________�����Ҫ˵��������������ˮ��ԭ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ��ɽ��ѧ��һ5���¿���ѧ�Ծ����������� ���ͣ�������
��ѡ���⣬10�֣���ij��̬����̼85.7%������14.3%���ڱ�״���µ��ܶ���2.5 g/L��������ʹ���Ը��������Һ����ˮ��ɫ��
(1)��������ʽΪ_______________________��
(2)д�����ĸ���ͬ���칹��Ľṹ��ʽ�� __________________________________��
��44.8 gͭ��140 mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ⣬������NO��NO2��������ڱ�״���µ����Ϊ11.2L��ش�
(1)����£�NO2�����Ϊ L
(2)������������ȫ���ͷź�����Һ�м���V mL a mol/L��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת���ɳ�������ԭ������ҺŨ��Ϊ �������ʽ��
(3)��ʹͭ�����ᷴӦ���ɵ�������O2��H2Oȫ��ת��ΪHNO3������ҪO2 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ͼ���ģ ���ͣ������
| ||
| ||
| ������ | �ܶ�/g?cm-3 | �е�/�� | �ܽ��/100gˮ |
| ������ | 0.810 | 118.0 | 9 |
| ������ | 1.049 | 118.1 | �� |
| ���������� | 0.882 | 126.1 | 0.7 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com