
| |||||||||||||||||||||||||||||||||||
|
�����𰸣��������� ������������(1)�⣬�ĸ�ѡ����A��B��D������ѧ��ѧ�̲���ѧ����Ϥ�ġ���Ȫ��ʵ�������Լ���ֻ��B�����O2��ˮ�е��ܽ��̫С�������γ��㹻��ѹǿ������γ���Ȫ���ʴ�ΪB�� ������(2)�����(1)С����ȣ���ԭ���ϼ�������֮�����в�֮ͬ��������֮���Ǿ�����ͨ���γ�һ����ѹǿ����γ���Ȫ����֮ͬ����ͼ��ͨ����С��ƿ��ѹǿ�γ�ѹǿ���ͼ����ͨ��������ƿ��ѹǿ�γ�ѹǿ������ĸ�ѡ�ֻ��Dѡ���ܲ���������CO2���壬ʹ��ƿ�ڵ�ѹǿ��������Һ��ѹ����ƿ��������Ȫ����ΪD�� ������(3)����ͨ�����γ���Ȫ���ܵ�ԭ������˹������ܽᣬͼ������Ϊ��ƿ������ѹǿͻȻ��С������ѹǿ���γɡ���Ȫ����ͼ�����෴������Ϊ�²���ƿ������ѹǿ���������ѹǿ���γɡ���Ȫ�������뵽��ʵ�����У���������Ȫ���롰��ɽ������������ͼ��ԭ�������Ƶ�ԭ��������ģ� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
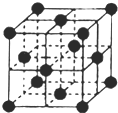
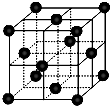 ���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]��
���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?������ģ����֪���صĽṹʽΪ��
��2013?������ģ����֪���صĽṹʽΪ��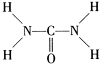 ���ؿ��������л����ʣ���Ҫ�������������������غ���������ѧʽΪ[Fe��H2NCONH2��6]��NO3��3��
���ؿ��������л����ʣ���Ҫ�������������������غ���������ѧʽΪ[Fe��H2NCONH2��6]��NO3��3��| 234 |
| ��NA |
| 234 |
| ��NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������һ���ºϳɵ����������Ϊ��������ṹ����̼�ܽṹ��ͼ��ʾ
��������һ���ºϳɵ����������Ϊ��������ṹ����̼�ܽṹ��ͼ��ʾ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
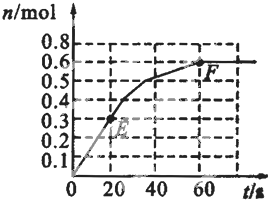 373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A ��g��?2B ��g������������B�����ʵ����仯��ͼ��ʾ
373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A ��g��?2B ��g������������B�����ʵ����仯��ͼ��ʾ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �һ��������ǵ�������������Ҫ�ĺϳ�����֮һ����ṹ��ʽ��ͼ��ʾ
�һ��������ǵ�������������Ҫ�ĺϳ�����֮һ����ṹ��ʽ��ͼ��ʾ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com