
��15�֣�����ʯ���ʵĸ���Ʒ����������(Na2SiF6)���Ʊ���ʯ(Na3AlF6)������ʯ�ǵ���������ۼ�,�ɽ������������۵㡣��ͼ�ǹ�ҵ����������ͼ��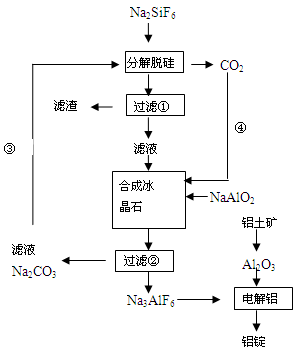
��1����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ����������__________�����ڣ���һ����Ӧ�ķ���ʽ______________________________________________________________
��2�����Ʊ������������ι��˲��������˲����ٵ���Һ��________��Һ��������________ ��
��3���ֽ��ѹ�ͺϳɱ���ʯ��ѧ��Ӧ����ʽ�ֱ�Ϊ��_________________��____________________��
��4�����չ����Тۺܵ͢�Ŀ����_____________________��̼���ƺͶ�����̼�Ƿ��� ��
��5�����Al2O3��Alʱ��I=200kA��һ����Al 1��430 t�����Ч���Ƕ��٣�
��1���� Al2O3+2NaOH=2NaAlO2+H2O
��2��NaF SiO2
��3��2Na2CO3+Na2SiF6=SiO2��+2CO2��+ 6NaF 6NaF +NaAlO2+2CO2=Na3AlF6��+2Na2CO3
��4��Na2CO3��CO2ѭ��ʹ�� �������ã�������ľ��貹��
��5��88��7%
���������������1����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ�������̣����ȼ�������NaOH��Һ��ʹAlת��ΪAlO2-,�������Һ�м���������������ᣬ��ʱAl��ΪAl3+��Ȼ����������İ�ˮ�õ�Al(OH)3������������˳�����ϴ�Ӹɾ�����ɡ��������Al(OH)3�õ�������Al2O3�������Ҫ�������ڡ���һ����Ӧ�ķ���ʽAl2O3+2NaOH=2NaAlO2+H2O����2�����Ʊ������������ι��˲�����������ͼ��֪���ڹ��˲����ٵ���Һ��NaF��������SiO2����3���ֽ��ѹ軯ѧ��Ӧ����ʽ��2Na2CO3+Na2SiF6=SiO2��+2CO2��+ 6NaF���ϳɱ���ʯ��ѧ��Ӧ����ʽ�ֱ�Ϊ��6NaF +NaAlO2+2CO2=Na3AlF6��+2Na2CO3 ����4�����չ����Тۺܵ͢�Ŀ����Na2CO3��CO2ѭ��ʹ�ã�������ʵ������ʡ����ܡ��������ɵ������ķ���ʽ��֪��������̼���ƺͶ�����̼ǡ����ȫ��Ӧ������ʵ��Ӧ��ʹ�������ʵ������ʲ�����100%�����Ծ��貹�䡣��5��һ����Al 1��430 t��n(e-)=1��430��106g��27g/mol��3=1��6��105mol������Q=1��6��105��6��02��1023��1��6��10-19=1��54��1010,���ĵĵ���n(e-)=2��105��3600��24=1��73��1010�����Ե��Ч���ǣ�1��54��1010��1��73��1010����100%=88��7%��
���㣺�������ʵ��Ʊ��������ķ��롢��ѧ����ʽ����д�����Ч�ʵ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������и�װ��ͼ�������У�����ȷ����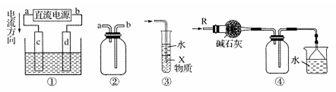
| A��װ�â��У�dΪ������cΪ���� |
| B��װ�âڿ������ռ�H2��CO2��Cl2��NO2������ |
| C��װ�â���X��Ϊ���Ȼ�̼�����������հ������Ȼ��⣬����ֹ���� |
| D��װ�âܿ����ڸ���ռ������������ն���İ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������刺����ڻ���ƽ�����ʵ���ҿ���NaClO3��ԭ����ȡ(���������ܽ����ͼ)����ʵ���������£�

��1�����������ȷֽ����ɸ������ƺ��Ȼ��ƵĻ�ѧ����ʽΪ ��
��2��80��ʱ��ȡҺ��ȴ��0����ˣ���������Ҫ�ɷ�Ϊ ��д��ѧʽ����
��3����Ӧ���м����Ȼ�隣�����Һ������Ӧ�����ӷ���ʽΪ ��
��4����֪��2NH4ClO4  N2��+2O2��+Cl2��+4H2O���ֿ��ṩ�����Լ���
N2��+2O2��+Cl2��+4H2O���ֿ��ṩ�����Լ���
a.����ʳ��ˮ b.ŨH2SO4 c.NaOH��Һ d.Mg e.Cu f.Fe
������ͼװ�öԸ�������ȷֽ����������������зֲ����ջ��ռ���

��E���ռ�������������� (�ѧʽ) ��
��װ��D�����ÿ����� ��
��A��B��C��ʢ�ŵ�ҩƷ���ο����� (ѡ��:��)��
��. a b d ��. c b e ��. b c f
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧС���������������������װ��(����ͼ)���û������Ʊ�����ϩ��
��֪��
| | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
����������SnSO4�������Ȼ�����SnCl4��������ӡȾ�͵�ƹ�ҵ��
��1��ij�о�С�����SnSO4�Ʊ�·�����£�
��֪�����������£�����ˮ��Һ����Sn2����Sn4��������Ҫ������ʽ��Sn2���ױ�������SnCl2����ˮ�⡣
��SnCl2���ܺ����Sn�۵������� ��
�ڲ�������õ��IJ����������ձ���� �����������Ҫϴ�ӹ���SnO�к��е����ʣ�����SnO�е�Cl���Ƿ�ϴ�Ӹɾ��IJ���Ϊ ��
�۲�����漰���IJ����У�a.���� b.ϴ�� c.����Ũ�� d.��ȴ�ᾧ e.���¸��������ȷ�IJ���˳��Ϊ ��
��2��ʵ������������װ�ã������ڵĽ���������﴿����������ȡ��ˮSnCl4��SnCl4�۵㣭33�棬�е�114.1�棬����ʪ��������ˮ�⣩���˷�Ӧ���̷ų��������ȡ�
��װ��C��Ӧ�����Լ�Ϊ ������E������Ϊ ��
�ڷ�Ӧ��ʼ����SnCl4ʱ��������Ϩ�� ������ĸ��ţ����ľƾ��ƣ������� ��
�۸�ʵ��װ������д���ȱ�ݣ��Ľ��ķ����ǣ������������Լ�������λ�õȣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��9�֣�ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽�����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�顣��ش�������⡣
��1��Ϊ��̽��SO2�ܷ�ʹƷ����ɫ����ͬѧѡ������ȷ��ҩƷ�����������ͼ��ʾʵ��װ�ã���ָ��ʵ��װ��ͼ����еIJ�����֮����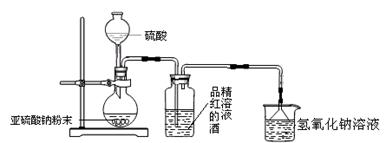
�� ��
�� ��
��2����ͬѧѡ������ȷװ�ú�ʵ���п��ƶ��������Դ�Լÿ��3�����ݵ��ٶ�ͨ��Ʒ��ľƾ���Һʱ������һСʱ��Ʒ���Բ���ɫ��Ϊ�ˣ�����ΪʹƷ���ˮ��Һ��ɫ���������� ��
��3����ͬѧ��һ��ʵ�����£�ȡ������ͬŨ�ȵ�Ʒ��ˮ��Һ����֧�Թ��У��ٷֱ���������������ƹ�������������ƹ��壬��֧�Թ��е�Ʒ�춼��ɫ�����ó����ۣ�ʹƷ����ɫ�����϶���HSO3-��SO32-������Ϊ���Ľ����Ƿ���ȷ �� �������� ��
��4����������װ��̽��SO2��ijЩ��ѧ���ʡ�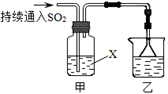
��װ���ҵ������� ��
����XΪNa2S��Һ���۲쵽��Һ�г��ֵ���ɫ���ǣ�˵��SO2���� ��
a�������� b����ԭ��
c��Ư���� d.���ȶ���
�����Լ�XΪCa(ClO)2��Һ���ɹ۲쵽��ɫ�������ɣ���ɸù��̵����ӷ���ʽ�� Ca2��+
Ca2��+ ClO��+
ClO��+ SO2+
SO2+ H2O��
H2O�� ��+
��+ Cl��+
Cl��+ SO42��+
SO42��+ ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣������ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3����һ�ֻ���ԭ�ϣ�������Ϊ��ɫҺ�壬������ˮ���е�Ϊ142.4�棬�ܶȱ�ˮС��ijʵ��С����������װ�úϳ������ѣ�����װ�þ���ȥ������������Ҫ��ӦΪ��2CH3CH2CH2CH2OH CH3CH2CH2CH2OCH2CH2CH2CH3+H2O
CH3CH2CH2CH2OCH2CH2CH2CH3+H2O
ʵ��������£����ݻ�Ϊ100mL��������ƿ�н�5mLŨ���ᡢ14.8g�������ͼ�����ʯ��Ͼ��ȣ��ټ��Ȼ���һ��ʱ�䣬�ռ����ֲ�Ʒ�����Ƶõ������ѡ��ش��������⣺
��1���ϳɴֲ�Ʒʱ��Һ���Լ�����˳���� ��
��2��ʵ��������ˮӦ�� �ڳ�ȥ���a����b������
��3��Ϊ��֤��Ӧ�¶Ⱥ㶨��135�棬װ��C����ʢҺ�������е���������Ϊ ��
��4������ʱ��������¶ȹ��ߣ���Ӧ���Һ���ڣ�д����NaOH��Һ�����ж�β�������ӷ���ʽ ��
��5���õ��������Ѵֲ�Ʒ������8 mL50%�����ᡢ10 mLˮ��ȡϴ�ӡ��ò�������Ҫ�����ڹ����β��ʵ�ʵ���������ձ����������� ��������ʹ��ǰ��Ҫ ��
��6������������л�������ˮ�Ȼ��Ƹ�����˺��ٽ��� ����������ƣ����Ƶõ������ѡ�
��7����ʵ�����յõ�6.50g�����ѣ��������ѵIJ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʵ�黯ѧ��
�ױ��������Ʊ�������ķ�Ӧԭ�����£�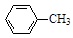 + 2KMnO4
+ 2KMnO4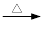
 +KOH+2MnO2��+H2O
+KOH+2MnO2��+H2O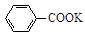 + HCl
+ HCl
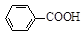 +KCl
+KCl
ʵ��ʱ��һ�����ļױ���KMnO4��Һ����ͼ1װ���У���100 ��ʱ�� ��Ӧһ��ʱ�䣬��ֹͣ��Ӧ�������������̷����������ͻ���δ��Ӧ�ļױ���

ͼ1��������װ�� ͼ2����װ��
��ʵ������ʵ�ֲ���������IJ��������� ���ձ��������������Ϊ ��
�������Һ����ɫ��Ҫ�ȼ���������أ�Ȼ���ټ���Ũ�����ữ�����˲�������ֵ�Σ���� ��
���ڲ������У�����ǰ�������ȴ��Һ����ԭ���� ����ͼ2��ʾ������ϣ�Ӧ�ȶϿ� ֮�����Ƥ�ܡ�
�ȴ��Ȳⶨ����ȡ1.220 g��Ʒ�����100 mL��Һ��ȡ����25.00 mL��Һ�����еζ� ������KOH���ʵ���Ϊ2.4��10��3 mol����Ʒ�б�������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ�Ȼ���������������ϵ������ӡȾ��ýȾ����Ⱦ�ϻ�ԭ������������ұ��ҽҩ���������ҵ��һʵ��С��ģ�¹�ҵ������ȡ�Ȼ����������װ������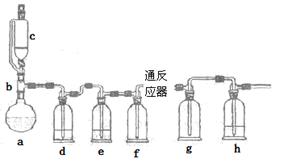
ͨ������������Ͽ�֪��
���ڳ�������500��ʱ�����봿��������Cl2��Ӧ������FeCl2�����¶Ƚϵ�ʱ������FeCl3��
��FeCl3�۷е�ͣ���������
����������Ϣ�ش���ص�����
��1��abc������ϳ���ȡ������װ�ã���Ӧ������ȣ���д��a�������������Ļ�ѧ��Ӧ����ʽ ��
��2��d��eʢװ��ҩƷ�ֱ��� �� ��f��g�������� ��
��3����Ӧ��ΪӲ�ʲ����ܣ�����������������500�����ҷ�Ӧ��
�ٻ�ѧ��Ӧ����ʽΪ
��ʵ�����˳���ǣ���װ������ ��װ��ҩƷ�� �� ��ֹͣ���ȡ��ر�c�Ļ�����
��4��ʵ��С���¼��ʵ���������£�
| | �۲쵽�IJ������� |
| ��һ��ʵ�� | ��Ӧ�����а�������ɫ���塢gƿ�а����ͻ���ɫ���� |
| �ڶ���ʵ�� | ��Ӧ��������ɫ���壬gƿ�к���ɫ���̺ͻ���ɫ���� |
| ������ʵ�� | ��Ӧ��������ɫ���壬gƿ�л���ɫ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com