
����Ŀ��ʯ���ѻ��ɵõ��л���(CH3)2C===C(CH3)2(������A��ʾ)��
��1��A��ϵͳ����Ϊ________��A��ͨ��״���³�________(�������Һ���̡�)̬��������ΪA����ij�ֹ�����ʹ��ˮ��ɫ����д����صĻ�ѧ����ʽ__________________________��
��2��A��Br2�ļӳɲ���B��NaOH���Ҵ���Һ���ȿ����ɶ�ϩ��C����C�Ľṹ��ʽΪ____________________________________________________________��B����C�ķ�Ӧ����Ϊ________��
��3��C��һ����Br2��Ӧ��������D��E��G����D��HBr�ļӳɲ���ֻ��F����F�Ľṹ��ʽΪ______________________________________________________��
���𰸡� 2,3-����-2-��ϩ Һ (CH3)2C===C(CH3)2 + Br2 ![]() (CH3)2CBrCBr(CH3)2
(CH3)2CBrCBr(CH3)2 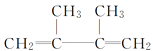 ��ȥ��Ӧ
��ȥ��Ӧ 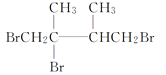
��������(1)����̼̼˫�����������4��C��̼̼˫������2��3��C�м䣬2��3��C����2������A��ϵͳ������Ϊ2��3-����-2-��ϩ��A���ں���6��̼ԭ�ӵ�����������ΪҺ�壬A�к���̼̼˫�����ܹ�����ˮ�����ӳɷ�Ӧ����Ӧ�ķ���ʽΪ��(CH3)2C=C(CH3)2 + Br2 �� (CH3)2CBrCBr(CH3)2�ʴ�Ϊ��2��3-����-2-��ϩ��Һ��(CH3)2C=C(CH3)2 + Br2 �� (CH3)2CBrCBr(CH3)2��
(2)BΪ(CH3)2CBrCBr(CH3)2���������NaOH���Ҵ���Һ���ȿ����ɶ�ϩ����Ϊ��ȥ��Ӧ������CΪ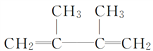 ���ʴ�Ϊ��
���ʴ�Ϊ��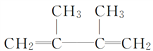 ����ȥ��Ӧ��
����ȥ��Ӧ��
(3)C��һ����Br2��Ӧ��������D��E��G����D��HBr�ļӳɲ���ֻ��F��˵��D�ṹ�Գƣ���C����1��4�ӳ�����D����FΪ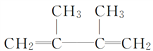 ���ʴ�Ϊ��
���ʴ�Ϊ��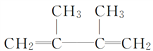 ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ͼ�жϣ�����������ȷ����
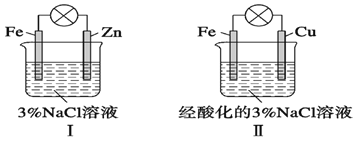
A. ��������������������
B. �������и�����Ӧ����Fe��2e��=Fe2��
C. ��������������Ӧ����O2��2H2O��4e��=4OH��
D. �������зֱ��������K3[Fe(CN)6]��Һ��������ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ͼ��ά����A�ķ��ӽṹ��ά����A�еĺ����������� _________�������ƣ���ά����A�ķ���ʽ�� ___________��1molά����A�������_______mol�巢���ӳɷ�Ӧ��
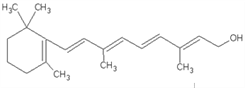
��2����������������ͬ���칹�����______________����ͬϵ�����_____________�����ڴ�����____________���˴Ź����������շ���4�����������Ϊ1��2��2��3����_____________��
��CH3CH2CH2CH3 ��CH3CH2CH3 ��CH3CH2OH ��CH3COOH ��CH3CH2COOH ��![]() ��
��![]() �� CH2=CHCH=CH2 ��CH��CCH2CH3
�� CH2=CHCH=CH2 ��CH��CCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2 A(g)��B(g)![]() 3C(g)��4D(g)��Ӧ�У���ʾ�÷�Ӧ���������ǣ� ��
3C(g)��4D(g)��Ӧ�У���ʾ�÷�Ӧ���������ǣ� ��
A. ��(A)��0.5mol/(L����) B. ��(B)��0.3mol/(L�� s) C. ��(C)��0.8mol/(L����) D. ��(D)��1mol/(L����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
��1��Ŀǰ������ʵ�õ�����ˮ��������Ҫ����֮һ�����������ǽ���ˮ�������������������ȴ���øߴ��ȵ�ˮ���ɴ˿��ж�������__________���������仯����ѧ�仯����
��2��ʵ������MnO2��Ũ����Ϊԭ���Ʊ��������÷�Ӧ�Ļ�ѧ����ʽΪ________________________________��������������_______��ԭ����________��β�����������ӷ���ʽΪ______________________________��
��3����ҵ���Ʊ�Ư�۵Ļ�ѧ����ʽ___________________________________��
��4����ʵ�����Ƶ�����������Ƶ���ˮ���ж����������÷���ʽ��ѧʽ�ش��������⣺
����ˮ�μ���ɫʯ����Һ�У��ȱ�����ɫ______________________________����ѧ����ʽ�����У�������ɫ����Ϊ��_________����ѧʽ�����ɾ���Ư���ԣ�
����ˮ������������Һ�У��а�ɫ��������__________________________�������ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol��L-1������ֱ�ΪVa��Vb��HA��Һ��BOH��Һ����ͬ����Ȼ�ϣ��ұ���Va+Vb=100mL��Va��Vb����Һ��pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
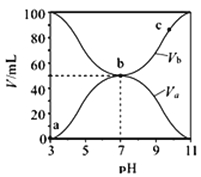
A. Ka(HA)��1��10-6
B. c��ʱ�� 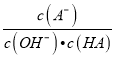 ���¶����߶�����
���¶����߶�����
C. a��c������ˮ�ĵ���̶�ʼ������
D. b��ʱ��c(B+)=c(A-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Fe2+�pNO3-�pNH4+�pFe3+��H+- H2O������,�ֱ�����һ��������ԭ��Ӧ�еķ�Ӧ���������,���������������( )
A. ����1mol NO3-�μӻ�ԭ��Ӧ,��ת��8mole- B. ��ԭ����ΪNH4+
C. �������뻹ԭ�������ʵ���֮��Ϊ8:1 D. �÷�Ӧ��Fe2+������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.01mol��L-1 �Ĵ�����Һ�������������1mol��L-1������Ũ�ȵ����ӣ���һ��ʱ����ʼ�ձ��ּ�С���Ƶ���
A. c(H+) B. c(CH3COO-)
C. c(H+)/c(CH3COOH)�ı�ֵ D. c(CH3COOH)/c(H+)�ı�ֵ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com