
| A��C��һ����H2SO4 |
| B�����ʵ���Ũ����ȵ�A��B����Һ��pH��С��ϵ��ǰ��С�ں��� |
| C��B������ˮ�п����ɳ��� |
| D����A��B����Һ��pH=9��������Һ����ˮ�������OH-�����ʵ���Ũ��֮��Ϊ1��104 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��CH2�TCH2+H-OH
| |||
B��H2+Cl2
| |||
C��2CH3-CH2-OH+O2
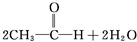 | |||
D��CH3-CH3+2Cl2
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����V�����仯����㷺Ӧ���ڹ�ҵ�����²��Ϻ�����Դ������
����V�����仯����㷺Ӧ���ڹ�ҵ�����²��Ϻ�����Դ������| ����SO2 |
| I |
| ����O2 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ��������a��b��c��d |
| B�����Ӱ뾶��A��n+1��+��Bn+��C��n+1��-��Dn- |
| C�����������ԣ�A��n+1��+��Bn+���ӻ�ԭ�ԣ�C��n+1��-��Dn- |
| D�����ʻ�ԭ�ԣ�B��A ���������ԣ�D��C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ������������Ϊ2��Ԫ��һ������Ԫ�����ڱ��ڢ�A�� |
| B��L�������Ϊ����������Ԫ����������������Ԫ��ԭ�ӵ�L���������ͬ |
| C������������Ԫ��X��Y���γ�XY2�ͻ������X��Y ��ԭ������֮�����Ϊ1��2 |
| D��M�������Ϊ��������������Ԫ����������������Ԫ��ԭ�ӵ�M���������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NH3?H2O |
| B��AlCl3 |
| C��CH3COOH |
| D��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ݢܢ� | B���ڢۢܢ� |
| C���ڢۢݢ� | D���٢ݢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com