
��16�֣� I��ʵ�����г���BaSO4�������ⶨBaCl2��nH2O�е�Ba�ĺ�����Ҫ�������£�
�� ���մ����������أ��Ƶ�����������
�� ��ȡBaCl2��nH2O�����ձ����ܽ⣬�����ᴦ��
�� ��һ��Ũ�ȵĹ���������뱵���еõ���ɫ����
�� ���ã�
�� ����������ֽ���ڴ������У����ƾ���Ƽ���̼��������800-850�����������أ�����
�� ����
��ش��������⣺
��1��ʡ�Եڢٲ��С����մ����������ء����ܵ��¼�����_ ______���ƫ�ߡ��������䡱��ƫ�͡�����
______���ƫ�ߡ��������䡱��ƫ�͡�����
��2���ڢܲ����ú�IJ����� ��
��3���ڢݲ������¶Ȳ�����900�棬����Ϊ_______________________��
II��ijͬѧ�ð�ˮ����һ������SO2�����պ���Һ�п��ܺ���OH-��SO32-��SO42-�� HSO3- ���������е������֡�
��4��д����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽ��
��5����֪����������һ�������ˮ������ѡ����������Լ����ձ����Թܡ�����������ͷ�ιܣ� 2 mol/L���ᡢ2 mol/L���ᡢ1 mol/L�Ȼ�����Һ��l mol/L����������Һ��Ʒ����Һ������ˮ�������ʵ��̽�����պ���Һ���Ƿ����SO32-��HSO3-����ʵ�������Ԥ�ڵ�ʵ������ͽ��������±��С�
| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թ�1�У��μӹ���lmol/L�Ȼ�����Һ�� | �������ֻ��ǣ�����Һ�в�����SO32-�� �����ֻ��ǣ�����Һ�п��ܺ���SO32-�� |
| ����2�������ֻ��ǣ�����һ��ʱ����ϲ���Һ�����Թ�2�С����Թ�1�м�������ˮϴ�ӳ��������ú���ȥ�ϲ���Һ���ټ��� �� | |
| ����3�� | |
��16�֣�
��1��ƫ�� ��2�֣� ��2�����ˡ�ϴ�ӳ�����2�֣�
��3�����ᱵ�ᱻ̼��ԭ���������ᱵ�ᷢ���ֽ⡱����2�֣�
��4��NH3��H2O + SO2 = NH4+ + HSO3- ��2�֣�
��5��ʵ����� Ԥ����������� ����2�� 2mol/L���ᡣ��1�֣� ���������������壬��֤����Һ�д���SO32-��
�������壬����SO32-����2�֣�����3�����Թ�2�м������2mol/L���ᣬ�ٵ���2��Ʒ�졣
�����Թ�2�м������lmol/L����������Һ����3�֣���ɫ��ȥ�������HSO3-����ɫ����ȥ������HSO3-��
���ֻ��ǣ������HSO3-�������ֻ��ǣ�����HSO3-����2�֣�
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ��������������̼����ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��CO2��SO2���ش��������⣺
��1��I��II��III��IVװ�ÿ�ʢ�ŵ��Լ� ��I ��II ��III ��IV ���������й��Լ����������ո��ڣ���
A��Ʒ����Һ B��Ca��OH��2��Һ C����ˮ��Һ
��2����˵��SO2������ڵ������� ��
��3��ʹ��װ��III��Ŀ���� ��
��4��ʹ��װ��IV��Ŀ���� ��
��5��ȷ��������ϩ�������� ��
��6����д��IIװ�÷����Ļ�ѧ����ʽ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ��̨һ�и�һ��ѧ�ڵ������¿���ѧ�Ծ����������� ���ͣ������
��֪A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�һ�־��й���ζ������E����ϳ�·������ͼ��ʾ��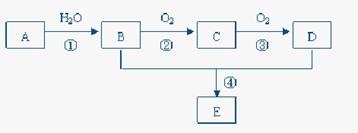
��ش��������⣺
��1��д��A�ĵ���ʽ ��
��2��B��D�����еĹ��������Ʒֱ��� �� ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
�� ��
��4����ѧ������ȤС��ѧ����ʵ��������ȡ��A�г����������Ķ���������ʦ�����������������ͼʵ����ȷ�����������������A��SO2���ش��������⣺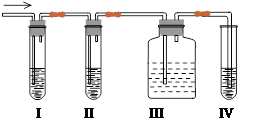
��I��II��III��IVװ�ÿ�ʢ�ŵ��Լ���I ��II ��III ��IV ��������ĸ��
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
��ʹ��װ��III��Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��16�֣�ʵ������ȡ��ϩ��װ������ͼ��ʾ�������ͼʾ�ش��������⣺

��1���÷�Ӧ��������_________________________��
ʵ��������ϩ�Ļ�ѧ����ʽΪ_________________________________________________
��2��ij��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ��
�����Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش�����
���⣺

�١�I��II����IVװ�ÿ�ʢ�ŵ��Լ���I II �� IV____ �����Լ�����ţ���
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
�ڡ���˵��SO2������ڵ�������_____________________________________________��
�ۡ�ʹ��װ��II��Ŀ����_____________________________________________________��
�ܡ�ʹ��װ�â��Ŀ����_____________________________________________________��
�ݡ�ȷ��������ϩ��������____________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�챱���и߶�12���¿���ѧ�Ծ� ���ͣ������
��28�֣���ϩ����Ҫ�Ļ���ԭ�ϣ����Ʊ��ܶ��л��ʵ������ȡ��ϩ��װ������ͼ��ʾ��
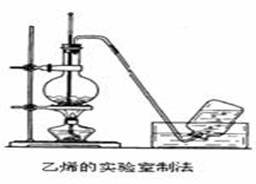
��1����ʵ������ȡ��ϩ�Ļ�ѧ��Ӧ����ʽΪ_______________________________________���ڴ˷�Ӧ����______________��Ӧ����ƿ�м������Ƭ��������______________________
��2��ij��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ��������ͼʵ��װ�ã���ȷ�����������������C2H4��SO2���ش��������⣺
�١�I��II����IVװ�ÿ�ʢ�Ų�ͬ���Լ������У�

I�� IV��_____�����Լ�����ţ�
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
�ڡ���˵��SO2������ڵ�������____________________________________
�ۡ�ȷ��������ϩ��������_____________________________________
(3) ʵ���п��Ը���ԭ�Ӻ˴Ź����ף�PMR���й۲쵽����ԭ�Ӹ����ķ������ȷ���л���
�Ľṹ���ú˴Ź����ķ������о�C2H6O�Ľṹ�����������Ϊ___________(���ֵ)����
ΪCH3CH2OH �����������Ϊ___________(���ֵ)����ΪCH3OCH3��
��4�����Ҵ�����ȡ������������д�йط�Ӧ����ʽ��ע����Ӧ��������
�����Ҵ�����������ȩ��_____________________________________________________
����ȩ������Cu��OH��2��Ӧ��_________________________________________________
�������ữ������ᣩ
���������Ҵ�������������_________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�캣��ʡ�߶���ѧ��������⣨һ����ѧ�Ծ� ���ͣ�ʵ����
( 14��)
(1)���������ڵ�����Ϊ���ʣ�����ȥ���и�������������������Լ���д�ں����ϣ�
��(�ױ�) ����(�Ҵ�) ���ױ�(��) ��
��2��ʵ�����ɵ�ʯ�е�̼���ƺ�ˮ��Ӧ��ȡ��Ȳ���÷�Ӧ�Ļ�ѧ����ʽΪ ��ʵ����Ϊ�˼�����Ӧ���ʣ����� ����ˮ����ʵ���в���������������ŵ���ζ��������_________________�����Լ������Գ�ȥ��
��3��ij��ȤС���ͬѧ��ʵ��������ȡ����ϩ�г������������������������������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺

1��I��II��III��IVװ�ÿ�ʢ�ŵ��Լ�����Ϊ ������ĸ��
��Ʒ����Һ ��NaOH��Һ ��Ũ���� ������KMnO4��Һ
A. �ܢڢ٢� B.�٢ڢ٢� C. �٢ڢ٢� D. �ܢڢ٢�
2����˵��SO2������ڵ������� ��
3��ʹ��װ��III��Ŀ���� ��
4��ȷ��������ϩ�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com