�����ҹ�����ӵ�����ʽϿ��������ƣ�����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
��1��������ȼ������ʱ����Ӧ��N
2��g��+O
2��g��?2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ�����ݻ�Ϊ2L���ܱ������г���10mol N
2��5mol O
2���ﵽƽ���NO�����ʵ���Ϊ2mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��
K=
0.11
0.11
��������������С�������λ���֣�
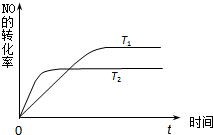
��2��һ������NO�����ֽ�Ĺ����У�NO��ת������ʱ��仯��������ͼ��ʾ������֪��T
1��T
2��
�ٷ�Ӧ 2NO��g��?N
2��g��+O
2��g��Ϊ������ȡ����ȡ���
����
����
��Ӧ��
��һ���¶��£��ܹ�˵����Ӧ 2NO��g��?N
2��g��+O
2��g�� �Ѵﵽƽ����ǣ�����ţ�
bc
bc
��
a�������ڵ�ѹǿ�������仯 b��NO�ֽ�����ʺ�NO���ɵ��������
c��NO��N
2��O
2��Ũ�ȱ��ֲ��� d����λʱ���ڷֽ�4mol NO��ͬʱ����2mol N
2��3���ٵ�����������ϡ��ȼ��ʱ��β���е���Ҫ��Ⱦ��ΪNOx������C
xH
y����������ԭNO
x���������������Ⱦ��
��֪��CH
4��g��+4NO
2��g��=�T4NO��g��+CO
2��g��+2H
2O��g����H
1=-574kJ?mol
-1CH
4��g��+4NO��g���T2N
2��g��+CO
2��g��+2H
2O��g����H
2CH
4��g��+2NO
2��g���TN
2��g��+CO
2��g��+2H
2O��g����H
3=-867kJ?mol
-1 ��H
2=
-1160 kJ?mol-1
-1160 kJ?mol-1
��
��ʹ�ô������Խ�����β������Ҫ�к��ɷ�һ����̼��CO���͵������NO
x��ת��Ϊ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ
��


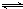 2NO��g��������ƽ������������������ʵ����ʵ�����������ƽ�ⳣ����ʽ���㣻
2NO��g��������ƽ������������������ʵ����ʵ�����������ƽ�ⳣ����ʽ���㣻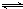 2NO��g����
2NO��g����


 ���ɴ���֪����Ӧ N2��g��+O2��g��?2NO��g��Ϊ������ȡ����ȡ���
���ɴ���֪����Ӧ N2��g��+O2��g��?2NO��g��Ϊ������ȡ����ȡ���