
��ѧ�Ҷ�һ̼��ѧ�����˹㷺���˵��о���ȡ����һЩ��Ҫ�ɹ���
(1)��֪��CO(g)+2H2(g)  CH3OH(g) ��H1��-90.1kJ/mol��
CH3OH(g) ��H1��-90.1kJ/mol��
3CH3OH(g) CH3CH=CH2(g)+3H2O(g) ��H2��-31.0kJ/mol
CH3CH=CH2(g)+3H2O(g) ��H2��-31.0kJ/mol
CO��H2�ϳ�CH3CH=CH2���Ȼ�ѧ����ʽΪ________��
(2)�������������Ϊ2L�ĺ����ܱ�����I��II�����У����ֱ����1molCO ��2mo1H2��������Ӧ��CO(g)+2H2(g) CH3OH(g) ��H1��-90.1kJ/mol�����������ķ�Ӧ�¶ȷֱ�ΪTl��T2��T3�Һ㶨���䡣����Ӧ�����е�5minʱH2�����������ͼ1��ʾ������ֻ��һ�������еķ�Ӧ�Ѿ��ﵽƽ��״̬��
CH3OH(g) ��H1��-90.1kJ/mol�����������ķ�Ӧ�¶ȷֱ�ΪTl��T2��T3�Һ㶨���䡣����Ӧ�����е�5minʱH2�����������ͼ1��ʾ������ֻ��һ�������еķ�Ӧ�Ѿ��ﵽƽ��״̬��

��5minʱ���������еķ�Ӧ�ﵽ��ѧƽ��״̬��������_______������ţ���
��0-5 min������I����CH3OH��ʾ�Ļ�ѧ��Ӧ����v(CH3OH)=_______��
�۵����������еķ�Ӧ���ﵽƽ��״̬ʱ,CO��ת������ߵ�������___________��������ţ���ͬ����ƽ�ⳣ����С��������_____________��
(3)CO�����ڹ�ҵұ���������ڲ�ͬ�¶�����CO ��ԭ���ֽ���������ﵽƽ���������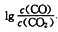 ���¶ȣ�T���Ĺ�ϵ��ͼ2��ʾ������˵����ȷ����_____������ĸ����
���¶ȣ�T���Ĺ�ϵ��ͼ2��ʾ������˵����ȷ����_____������ĸ����
a.��ҵ�Ͽ���ͨ�����߷�Ӧװ�����ӳ���ʯ��CO�Ӵ����������β����CO�ĺ���
b.CO���ڹ�ҵұ��������(Cr)ʱ����ԭЧ�ʲ���
c.��ҵұ������ͭ(Cu) ʱ��600����CO�������ʱ�1000����CO�������ʸ���
d.CO��ԭPbO2�ķ�Ӧ��H��0
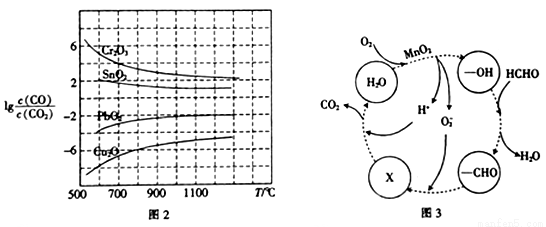
��4����ҵ��ˮ�к��м�ȩ���ó������ȩ�ķ�Ӧ������ͼ3��ʾ����X��ʾ��������_____���ܷ�Ӧ�Ļ�ѧ����ʽΪ_________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����3�¸߿�ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�������������Խϴ�Ũ�ȹ�����ǣ� ��
A. ����0.1mol��L��1Fe3������Һ�У�K����Mg2����I����NO3��
B. ʹ��̪��Һ������Һ��Na����Cl����SO42����CO32-
C. ����0.1mol��L��1Ca2������Һ�У�Na����K����CO32����Cl��
D. ̼��������Һ��K����SO42����Cl����H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ��������2����ѧ�Ծ��������棩 ���ͣ�ѡ����
���м�ͥ��ѧСʵ�鲻�ܴﵽԤ��Ŀ�ĵ���
A. ����������ʳ�üӵ��Σ���KIO3���к��е�
B. �ôס�ʯ��ˮ��֤�����к���̼����
C. �õ�Ƽ����������Ƿ��в�������
D. �ü����ס�ʳ�Ρ�ˮ��ɵ����ʵ��ܽ⡢����ʵ��?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ̩���и�����һ�ָ�ϰ������⣨һģ�������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��Ȳ����Ҫ�Ļ���ԭ�ϣ��㷺�����л��ϳɺ���Ȳ���ȡ�������Ȳ�ķ����ж��֣����ʯ���������ѽⷨ�ȡ�
(1)��Co(NO3)2���£���Ȳ�ɱ�50����Ũ����(���ᱻ��ԭΪNO2)��20~70��ʱֱ������ΪH2C2O4��2H2O��
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ________________________��
��ʵ�������������ѭ�����ö��������ģ��÷���ʽ˵����___________________��
(2)��ʯ��ԭ��Ϊ����ʯ�ͽ�����ʯ���ڵ�¯�����ɵ�ʯCaC2(��Ca3P2��CaS������)�� ��ʯ��ˮ��Ӧ����C2H4(��PH3��H2S������)��
����֪��̿�����������ƹ���ÿ����l g CaC2���壬ͬʱ����CO��������7.25kJ�� ��������÷�Ӧ���Ȼ�ѧ����ʽΪ_____________________________________��
����CuSO4��Һ������Ȳ���壬ȥ��PH3�ķ�Ӧ֮һΪ��4CuSO4+PH3+4H2O===4Cu��+H3PO4+4H2SO4��ÿȥ��1 mol PH3���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ__________��
�۷�ӦH2S(aq)+Cu2+(aq)===CuS(s)+2H+(aq)��ƽ�ⳣ��Ϊ________________��(��֪Ksp(CuS)=1.25��10-36��H2S��Kal=1��10-7��Ka2=1��10-13)
�ܵ�ʯ���������̼����ײ�������Ȳ���ȸߣ�ȱ����_______(��1��)��
(3)�����ѽⷨԭ��Ϊ��2CH4(g) C2H2(g)+3H2(g)��H��ʵ���ø÷�Ӧ��Kp(��ƽ���ѹ����Ũ�ȼ����ƽ�ⳣ������ѹ=��ѹ�����ʵ�������)���¶ȵĹ�ϵ��ͼ��ʾ��
C2H2(g)+3H2(g)��H��ʵ���ø÷�Ӧ��Kp(��ƽ���ѹ����Ũ�ȼ����ƽ�ⳣ������ѹ=��ѹ�����ʵ�������)���¶ȵĹ�ϵ��ͼ��ʾ��
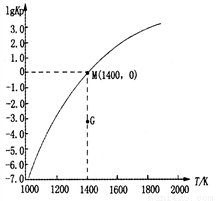
�ٸ÷�Ӧ�ġ�H________0(�>������=����<��)��
��ͼ��G��v(��)______v(��)(�>������=����<��)��
��M��ʱ��������������������ʵ���Ϊ1 mol������ѹP��n(CH4)��n(C2H2)��n(H2)֮��Ĺ�ϵΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ̩���и�����һ�ָ�ϰ������⣨һģ�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
NA���������ӵ�������ֵ������������ȷ���ǣ� ��
A. 9 g����ˮ��3H216O����������Ϊ6NA
B. ��״���£�22.4 L CCl4���еķ�����ĿΪNA
C. ���³�ѹ�£�16g�����й��ۼ���ĿΪ4NA
D. 1 L 0.1 mol��L-1��NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ��δ��������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���в�����װ���ܴﵽʵ��Ŀ�ĵ���
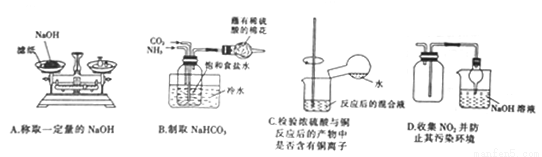
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����и�����ѧ�ڵڶ�������������ۻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ�ϳ��Ը�������Ҫ�ɷ�Ϊ�Ǹ�������FeCr2O4��������Al2O3��SiO2�����ʣ�Ϊ��Ҫԭ�������췯�ƣ�Na2Cr2O7����ijʵ��С��ģ������������ͼ��ʾ��
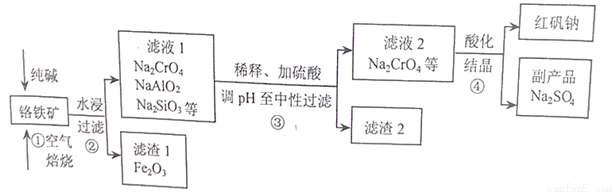
��1��FeCr2O4��Cr�Ļ��ϼ�Ϊ____________������ٱ���ʱ���õ���������Ϊ_________�������������Na2CrO4�Ļ�ѧ����ʽΪ________________________��
��2����������շ�Ӧ��������Ҫ���������������״̬����Ӧ���ʲżӿ죬����Ҫԭ��Ϊ________��
��3������ڹ���ʱ���õ��IJ���������________________��
��4������������Ὣ��Һ��pH�������ԣ���������2����Ҫ�ɷ���__________��__________��
��5�����������Һ2���������ữ�����У���Һ�ɻ�ɫ��Ϊ��ɫ����Ӧ�����ӷ���ʽΪ___________��
��6����ͼ��Na2Cr2O7��2H2O��Na2SO4���ܽ�����ߣ�������л�ø���ƷNa2SO4��ʵ���������Ϊ____________________��
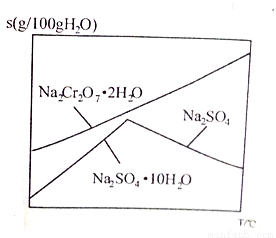
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������е�ʮ��У�ص���ѧ������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ����
A. �����£���0.01mol/LNH4HSO4��Һ�еμ�Na0H��Һ�����ԣ�c(Na+)>c(SO42-)>c(NH4+)> c(OH-)= c(H+)
B. Na2CO3��Һ��c(Na+)+ c(H+)=c(HCO3-)+c(CO32-)+ c(OH-)
C. ��Ũ�ȵ�NaClO��NaHCO3�����Һ�У�c(HClO)+c(ClO-)= c(HCO3-)+c(H2CO3)
D. ��ˮ�У�2c(Cl2)=c(ClO-)+c(Cl-)+c(HClO)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶�3��ѧҵˮƽ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й�����û�з�����ѧ�仯���ǣ� ��
A. ú��Һ�� B. �Ӻ�ˮ�еõ�������
C. ʯ����������� D. �Ӻ�������ȡ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com