
��ϩͨ����ˮ���������ˮ�ⶼ����ѧ�л���ѧ����Ҫ��Ӧ��
��1����д��ʵ��������ϩ�Ļ�ѧ����ʽ ��
��2����ʵ�����ѧϰС���ͬѧ����ϩ�������ͨ��100�������͵���ˮ��Լ0.2mol/L��ֱ����ˮ��ȫ��ɫ����ϸ�۲�õ�����ɫ��Һ����������������״Һ�Ρ�
������ˮ��ɫ�Ľ��ͣ�ͬѧ�������ֹ۵㣬һ����Ϊ������ȡ����Ӧ����һ����Ϊ�����˼ӳɷ�Ӧ������һͬ����ʵ�鲢�ش��й����⡣
����״Һ�δ��� �㣨��ϡ����¡�����
�����˵ó����ۣ��ⶨ��ɫ��Һ��pH�����жϸ÷�Ӧ��ȡ����Ӧ���Ǽӳɷ�Ӧ���������ǣ�
��
�۲����ɫ��Һ��pH=7������ϩ����ˮ��Ӧ�Ļ�ѧ����ʽΪ
��
��3�����ʵ�����������ˮ������������ӡ�
��ʵ���Լ���pH��ֽ�������顢NaOH��Һ��AgNO3ˮ��Һ�� ��
ʵ����������������������ͷ�ιܡ��Թܼ��ԹܼС� ��
��������ˮ��Ļ�ѧ����ʽΪ ��
�ж��������Ѿ���ȫˮ��������� ��
��ȡ������������ȫˮ������õ���Һ���Թ��У�����һϵ��ʵ�飬����������ɫ������˵��������ˮ������������ӡ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ӡˢ��·���������Ϻ�ͭ�����϶��ɣ�������ӡˢ��·ʱҪ����Һ��Ϊ����ʴҺ��
�ܽ�ͭ��
��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________;
��2��д��FeCl3��������Zn��Ӧ�Ļ�ѧ����ʽ��__________________________________;
��3����ʴҺ�þû�ʧЧ�������Ի������ã�
����Ҫ�õ�����ͭ�������Լ���ʵ�ֵ��ǣ�����ţ�
A������ B���� C������ D��ϡ���� E����
����Ҫ��ת��Ϊ�������Լ���ʵ�ֵ��ǣ�����ţ�
A������ B���� C������ D��ϡ���� E����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ط�Ӧ�����ӷ���ʽ��д��ȷ����
A������������������Fe(OH)3+3H+ = Fe3++3H2O
B������ͭ��Һ�����ԣ�Cu2+ + 2H2O == Cu(OH)2��+ 2H+
C����̼�������Һ�мӹ���ʯ��ˮ�����ȣ�NH4++OH�� NH3��+H2O
NH3��+H2O
D�����ữ�ĸ��������Һ����˫��ˮ��2MnO4��+6H++5H2O2 = 2Mn2++5O2��+8H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ�������ֵ��������������ȷ����
A����״���£�22.4L�����й��ۼ���ĿΪ19NA
B��26g��Ȳ�뱽�Ļ������������̼ԭ����Ϊ0.2NA
C���ڱ�״���£�2.24L��Ȳ�еĦҼ���Ϊ0.3NA
D��1 mol���������ӳ������Ҫ�������Ϊ6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���ܱ������з�������ʵ�����Na2O2�����NaHCO3���壬��ּ��Ⱥ���ȴ���ų����������塣���ж������е�ʣ������˵����ȷ����
A��ʣ�����ֻ��Na2CO3
B��ȡ����ʣ����������Һ����μ���ϡ���ᣬ������������
C��ʣ����������NaHCO3��NaOH�Ļ����
D��ʣ������ǵ����ʵ�����Na2CO3��NaOH�Ļ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ܹ�֤�����鹹�������������ʵ�� �� ��
A��������ĸ���������ͬ B��������ĸ����������
C�����������C-H��������� D�����ȼ���û��ͬ���칹��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͬ���칹���������л���ѧ���Ƿdz��ձ�ģ������л��ﻥΪͬ���칹����ǣ� ����CH2��CHCH3 ��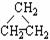 ��CH3CH2CH3 ��HC
��CH3CH2CH3 ��HC CCH3 ��
CCH3 ��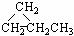 ��CH3CH��CHCH3
��CH3CH��CHCH3
A���ٺ͢� B���ٺ͢� C���ٺ͢� D���ݺ͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧ������ȷ����
A����ϩ���ӵĵ���ʽ 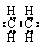
B����ԭ�ӵĽṹʾ��ͼ 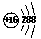
C�����۵Ļ�ѧʽ (C6H10O5)n
D���������ĵ��뷽��ʽ Fe2(SO4)3  = 2Fe2+ + 3SO42��
= 2Fe2+ + 3SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ�������ֵ��������������ȷ����
A�������£�1 mol Cl2������NaOH��Һ��ȫ��Ӧ��ת�Ƶĵ�����2NA
B�����³�ѹ�£�11.2 L CO2�к��еķ�������0.5NA
C����״���£�22.4 Lˮ����ԭ����Ϊ3NA
D�����³�ѹ�£�48 g O3��O2�Ļ����������ԭ����Ϊ3NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com