
(1)��ӦA(g)+B(s) C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
������___ ���� ����ѹǿ____ �� �����������ݻ�____ ��
�� ����A___ �� �� ����B____ �� �� ��������____ ��
��2����25�桢101kPa�£�1g����ȼ������CO2��Һ̬ˮʱ����55.6kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ___________________________________________��
��3���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������������ɵñ�ϩ��
��֪��C3H8(g) CH4(g)��HC
CH4(g)��HC CH(g)��H2(g) ��H1=156.6 kJ��mol��1
CH(g)��H2(g) ��H1=156.6 kJ��mol��1
CH3CH CH2(g)
CH2(g) CH4(g)��HC
CH4(g)��HC CH(g
) ��H2=32.4 kJ��mol��1
CH(g
) ��H2=32.4 kJ��mol��1
����ͬ�����£���ӦC3H8(g) CH3CH
CH3CH CH2(g)��H2(g) �ġ�H=
kJ��mol��1��
CH2(g)��H2(g) �ġ�H=
kJ��mol��1��
��4���±��е����ݱ�ʾ�ƻ�1 mol��ѧ�������ĵ�����(�����ܣ���λΪkJ��mol��1)��
|
��ѧ�� |
C��H |
C��F |
H��F |
F��F |
|
���� |
414 |
489 |
565 |
158 |
���ݼ������ݼ������·�Ӧ�ķ�Ӧ�ȡ�H��
CH4��g��+4F2��g����CF4��g��+4HF��g�� ��H=______________________
��1���ӿ졢�ӿ졢�������ӿ졢���䡢�ӿ� ��1��/ÿ�գ�
��2��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H���C889.6kJ��mol��1��4�֣�
��3��124.2��3�֣� ��4����1928 KJ/mol��3�֣�
����������1��������������Է�Ӧ���ʵ�Ӱ�졣����Ӧ���Ũ�ȡ������¶ȡ�����ѹǿ��ʹ�������������ܼӿ췴Ӧ���ʡ������������ݻ���ѹǿ��С����Ӧ���ʽ��͡�B�ǹ��壬����B����������Ӧ���ʲ��䡣
��2��ȼ������ָ��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������������֪��1mol������ȫȼ�շų���������55.6kJ��16��889.6 kJ�����Լ����ȼ���ȵ��Ȼ�ѧ����ʽΪCH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H���C889.6kJ��mol��1��
��3�������˹���ɵ�Ӧ�á���������٣��ڼ��õ�C3H8(g) CH3CH
CH3CH CH2(g)��H2(g)�����Ը÷�Ӧ�ķ�Ӧ����156.6 kJ��mol��1��32.4 kJ��mol��1��124.2 kJ��mol��1��
CH2(g)��H2(g)�����Ը÷�Ӧ�ķ�Ӧ����156.6 kJ��mol��1��32.4 kJ��mol��1��124.2 kJ��mol��1��
��4����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����414 kJ��mol��1��4��158 kJ��mol��1��4��489 kJ��mol��1��4��565 kJ��mol��1��4����1928 KJ/mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ����һ��2011��2012ѧ��߶�ģ���⻯ѧ���� ���ͣ�022
�ش��������⣺
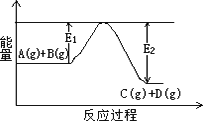
(1)��ӦA(g)��B(g)![]() C(g)��D(g)�����е������仯��ͼ��ʾ���жϸ÷�Ӧ��H________0(�������������������ȷ����)��
C(g)��D(g)�����е������仯��ͼ��ʾ���жϸ÷�Ӧ��H________0(�������������������ȷ����)��
(2)��Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)��CO(g)��H2O(g)����H1����34.0 kJ/mol
����(g)��CO2(g)��H2(g)����H2����7.0 kJ/mol
�����ķ���ʽΪ________���ڸ������£���̬CO2����̬H2������̬CO����̬H2O���Ȼ�ѧ����ʽΪ________��
(3)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N2H4)��ǿ������Һ̬˫��ˮ(H2O2)��
����0.4 molҺ̬�º�0.8 molҺ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7 kJ������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡʼ���ط����ѧ�߶���ѧ����ĩ�������ۻ�ѧ������������ ���ͣ���ѡ��
(1)��ӦA(g)+B(s) C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
������___ ��������ѹǿ____ �������������ݻ�____ ��
�ܼ���A___ �� �ݼ���B____ �� ��������____ ��
��2����25�桢101kPa�£�1g����ȼ������CO2��Һ̬ˮʱ����55.6kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ___________________________________________��
��3���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������������ɵñ�ϩ��
��֪��C3H8(g) CH4(g)��HC
CH4(g)��HC CH(g)��H2(g) ��H1="156.6" kJ��mol��1
CH(g)��H2(g) ��H1="156.6" kJ��mol��1
CH3CH CH2(g)
CH2(g) CH4(g)��HC
CH4(g)��HC CH(g ) ��H2="32.4" kJ��mol��1
CH(g ) ��H2="32.4" kJ��mol��1
����ͬ�����£���ӦC3H8(g) CH3CH
CH3CH CH2(g)��H2(g) �ġ�H= kJ��mol��1��
CH2(g)��H2(g) �ġ�H= kJ��mol��1��
��4���±��е����ݱ�ʾ�ƻ�1 mol��ѧ�������ĵ�����(�����ܣ���λΪkJ��mol��1)��
| ��ѧ�� | C��H | C��F | H��F | F��F |
| ���� | 414 | 489 | 565 | 158 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʼ���ط����ѧ�߶���ѧ�����п������ۻ�ѧ�Ծ����������� ���ͣ������
(16��)(1)��ӦA(g)+B(s) C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
������____________��������ѹǿ____________�������������ݻ�____________��
�ܼ���A____________�� �ݼ���B____________�� ��������____________��
��2����25�桢101kPa�£�1g����ȼ������CO2��Һ̬ˮʱ����55.6kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ___________________________________________��
��3���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������������ɵñ�ϩ��
��֪��C3H8(g)=CH4(g)��HC CH(g)��H2(g) ��H1="+156.6" kJ��mol��1
CH(g)��H2(g) ��H1="+156.6" kJ��mol��1
CH3CH CH2(g)= CH4(g)��HC
CH2(g)= CH4(g)��HC CH(g ) ��H2="+32.4" kJ��mol��1
CH(g ) ��H2="+32.4" kJ��mol��1
����ͬ�����£���ӦC3H8(g)=CH3CH CH2(g)��H2(g) �ġ�H= kJ��mol��1��
CH2(g)��H2(g) �ġ�H= kJ��mol��1��
��4���±��е����ݱ�ʾ�ƻ�1 mol��ѧ�������ĵ�����(�����ܣ���λΪkJ��mol��1)��
| ��ѧ�� | C��H | C��F | H��F | F��F |
| ���� | 414 | 489 | 565 | 158 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʼ���ط����ѧ�߶���ѧ�����п������ۻ�ѧ�Ծ��������棩 ���ͣ������
(16��)(1)��ӦA(g)+B(s) C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
������____________���� ����ѹǿ____________�� �����������ݻ�____________��
�� ����A____________�� �� ����B____________�� �� ��������____________��
��2����25�桢101kPa�£�1g����ȼ������CO2��Һ̬ˮʱ����55.6kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ___________________________________________��
��3���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������������ɵñ�ϩ��
��֪��C3H8(g)=CH4(g)��HC CH(g)��H2(g)
��H1=+156.6
kJ��mol��1
CH(g)��H2(g)
��H1=+156.6
kJ��mol��1
CH3CH CH2(g)=
CH4(g)��HC
CH2(g)=
CH4(g)��HC CH(g
) ��H2=+32.4
kJ��mol��1
CH(g
) ��H2=+32.4
kJ��mol��1
����ͬ�����£���ӦC3H8(g)=CH3CH CH2(g)��H2(g)
�ġ�H=
kJ��mol��1��
CH2(g)��H2(g)
�ġ�H=
kJ��mol��1��
��4���±��е����ݱ�ʾ�ƻ�1 mol��ѧ�������ĵ�����(�����ܣ���λΪkJ��mol��1)��
|
��ѧ�� |
C��H |
C��F |
H��F |
F��F |
|
���� |
414 |
489 |
565 |
158 |
���ݼ������ݼ������·�Ӧ�ķ�Ӧ�ȡ�H��
CH4��g��+4F2��g����CF4��g��+4HF��g�� ��H=______________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com