
ij��ȤС��̽��SO2���廹ԭFe3����I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��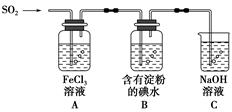
(1)SO2���廹ԭFe3���IJ�����________(�����ӷ���)���μӷ�Ӧ��SO2��Fe3�������ʵ���֮����________��
(2)����ʵ�鷽����������ʵ������ȡ����SO2����________��
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D��ͭ����ŨH2SO4
(3)װ��C��������____________________________________��
(4)��Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ���������________(�����)��
A���������� B��ʯ����
C��©�� D���ձ� E�������� F������
(5)������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3��������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�������KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ�м�����ϡ�����ữ��BaCl2��������ɫ������
������������������________��ԭ����__________________________
______________________________________________��
(6)�ܱ���I����ԭ������SO2��������_____________________________
___________________________________________��
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���Ҫ�������£�
(1)Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ��________��ҪʹMgSO4��ȫת��Ϊ�����������Լ�����ӦΪ________________��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����_____________��
(3)�Լ���ѡ��________��д���䷴Ӧ�����ӷ���ʽ_______��
(4)��ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
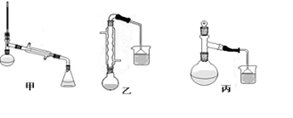
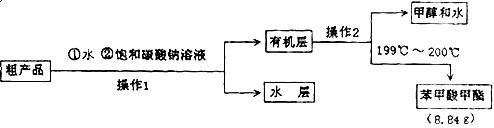
ij��ѧС���Ա�����Ϊԭ����ȡ������������й����ʵķе����Է������������
| ���� | �״� | ������ | ��������� |
| �е㣯�� | 64.7 | 249 | 199.6 |
| ��Է������� | 32 | 122 | 136 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������[(NH4)2SO4��FeSO4��6H2O]Ϊdz��ɫ���壬������ˮ�������ھƾ�����ˮ�е��ܽ�ȱ�FeSO4��(NH4)2SO4��ҪС��ʵ�����г��Է���мΪԭ�����Ʊ����䲽�����£�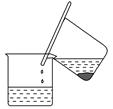
ͼ1
����1����м�Ĵ�����������м�����ȵ�̼������Һ�н��ݼ����Ӻ���ͼ1��ʾ������������岢ϴ�ӡ����
����2��FeSO4��Һ���Ʊ����������õ���м������ƿ�����������3 mol��
L��1H2SO4��Һ����������ַ�ӦΪֹ�����ȹ���(��ͼ2��ʾ)���ռ���Һ��ϴ��Һ��
ͼ2
����3����������淋��Ʊ���������FeSO4��Һ�м��뱥��(NH4)2SO4��Һ����������Ũ������ȴ�ᾧ�����ˡ��Ҵ�ϴ�Ӻ�õ���������茶��塣
��ش��������⣺
(1)����1��ͼ1���뷽����Ϊ________����
(2)����2����һ�����Բ���������___________________________________��
���ȹ��˵�������________________________________________________��
(3)����3����Ũ�������У���________ʱֹͣ���ȡ�����ˮ�Ҵ�ϴ�Ӿ����ԭ����______________________________________________________________��
(4)FeSO4��7H2O�ڳ�ʪ�Ŀ������ױ�������Fe(OH)SO4��3H2O��д���÷�Ӧ�Ļ�ѧ����ʽ________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ૼ������ȵõ�����ͪ���������������ϣ��ɵõ��˼������ӿ���ϩ���й�ʵ��ԭ�����������£� 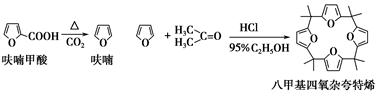
����1����Ʊ�
��Բ����ƿ�з���4.5 gૼ��ᣨ100 ��������ૼ�����133 �����ڣ�230��232 ����ڣ����ڴ��¶������ȣ�����ͼ��װ���������ȴ�����ʹૼ�������ۻ���Ȼ����ڼ���ǿ�ȣ��������У���ૼ������ȷ�Ӧ��ϣ�ֹͣ���ȡ�����ɫҺ��ૣ��е㣺31��32 �棬������ˮ����
����2��������˼������ӿ���ϩ�ĺϳ�
��25 mL��ƿ�м���2.7 mL 95%�Ҵ���1.35 mLŨ���ᣬ���ȣ��ڱ�ԡ������5 �����£�Ȼ��3.3 mL��ͪ��1.35 mLĻ��ҺѸ�ٵ�����ƿ�У���ֻ��ȣ���ԡ��ȴ�����õ�һ��ɫ��״���塣���ˣ�����3 mL��ˮ�Ҵ�ϴ�ӣ��ñ��ؽᾧ���ð�ɫ�ᾧ�˼������ӿ���ϩ��
��1������1���ô���ټ��ȣ�����ҪĿ����____________________________��
��2��װ��ͼ�м�ʯ�ҵ�������__________________________________________��
��ˮ�Ȼ��Ƶ�������________________________________________________��
��3������װ�����ñ���ԡ��Ŀ����_____________________________________��
��4���ϳɰ˼������ӿ���ϩ���������Ŀ����_________________________��
��5��ȷ�۲�ƷΪ�˼������ӿ���ϩ����ͨ���ⶨ�е㣬���ɲ��õļ�ⷽ����__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��˾ƥ�ֿ���ˮ�����������������Ƶá����Ʊ�ԭ�����£�

��˾ƥ��(����ˮ����)������������ˮ����˾ƥ�ֿɰ����²�����ȡ�ʹ�����
����1���ڸ����50 mLԲ����ƿ�м���2 gˮ���ᡢ5 mL��������5��Ũ���ᣬ��ʹˮ����ȫ���ܽ⡣
����2����ͼ��ʾװ��װ���������ͨˮ����ˮԡ�ϼ��Ȼ���5��10 min������ˮԡ�¶���85��90 �档
����3����Ӧ������ȡ�·�Ӧƿ����ȴ���ٷ����ˮ����ȴ���ᾧ�����ˡ���ˮϴ��2��3�Σ��������˵ôֲ��
����4�����ֲ���ת����150 mL�ձ��У��ڽ����¼���25 mL����̼��������Һ����ֽ��裬Ȼ����ˡ�
����5������Һ����10 mL 4 mol��L��1���ᣬ���裬���ձ����ڱ�ԡ����ȴ��ʹ�ᾧ��ȫ�����ˣ�������ˮϴ��2��3�Ρ�
(1)����1Ũ��������ÿ�����________��
(2)����2�У�������ͨˮ��ˮӦ��________�ڽ�(�a����b��)��
(3)����3����ʱ����ʱ��ֽ�ᴩ�ף�������ֽ���Ĵ�ʩ��______________________________________________________________��
(4)����4������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_____________________�����˵õ��Ĺ���Ϊ________��
(5)ȡ��������5��õľ������ʢ��5 mLˮ���Թ��У�����1��2��1%���Ȼ�����Һ��������Һ����ɫ���ɲ���________��������һ���������塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
SiO2��SO2��CO2����������������ǵĻ�ѧ���ʾ���һ���������ԣ�Mg��Na�Ļ�ѧ����Ҳ����һ�������ԡ�
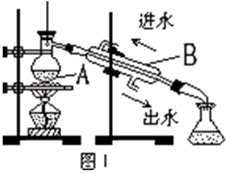
ij��ȤС������ͼ��ʾװ�ý���Mg��SO2��Ӧ��ʵ�顣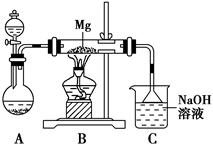
(1)ѡ����ȡSO2�ĺ����Լ�________(����)��
��ŨHCl����ŨH2SO4����Na2SO3���塡��CaSO3����
(2)����װ�û����Ż����Ż��ķ�����________________________________________��װ��C��NaOH��Һ��������___________________________________________________________
(3)��ͬѧ�Ʋ�Mg��SO2�ķ�Ӧ��Mg��CO2�ķ�Ӧ���ƣ���÷�Ӧ����ʽΪ_________________________________________��
��ͬѧ���Ʋ��ǣ�2Mg��3SO2 2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2
2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2 2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����
2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����
��֪��MgSO3��MgS������ˮ���������ᷢ�����ֽⷴӦ�ų����壻H2S����ͨ��CuSO4��Һ�г��ֺ�ɫ������
��ѡ�Լ���2 mol��L��1���ᡢ2 mol��L��1���ᡢ����ˮ��2 mol��L��1 NaOH��Һ��Ʒ����Һ������ʯ��ˮ��2 mol��L��1 CuSO4��Һ����������Ʒ��ѡ��
| ��� | ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ȡ������Ӧ�����ù������Թ��� | |
| �� | ���Թ��еĹ��������μ�____________���Թܿ����ϴ����ܵĵ���������������ͨ��ʢ��________���Թ��� | ���Թ��е�________�����ͬѧ�Ʋ���ȷ�����Թ��еĹ���δ��ȫ�ܽ⣬��________������ͬѧ�Ʋ���ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ƣ�NaClO2����һ�ָ�Ч��������Ư������֪��NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ���Ʊ��������ơ�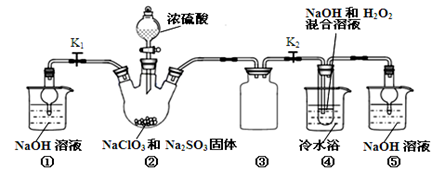
���������գ�
��1��װ�â��в���ClO2�Ļ�ѧ����ʽΪ ��װ�â۵������� ��
��2����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���� ���� ���ܵ���60�����õ���Ʒ��
��3��ȷ��ȡ��������������Ʒ10g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ��ClO2��+ 4I��+4H+ ��2H2O+2I2+Cl�����������û��Һ���250mL������Һ�����ƴ���Һ���õ��Ķ������������� ��
��4��ȡ25.00mL����Һ����2.0 mol/L Na2S2O3��Һ�ζ���I2 +2S2O32����2I��+S4O62�������Ե�����Һ��ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ ���ظ��ζ�2�Σ����Na2S2O3��Һƽ��ֵΪ20.00 mL������Ʒ��NaClO2����������Ϊ ��
��5��ͨ������˵��װ�â��ڱ�ʵ���е����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�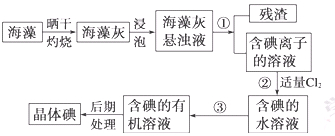
��1��ָ�������۵����ƣ� ���������г�������Cl2��Ŀ���� ��
��2����ȡ��Ĺ����У��ɹ�ѡ����Լ��� ( )
| A���ƾ� | B�����Ȼ�̼ | C������ | D������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com