
 ijѧ����NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ����������ʵ�飺
ijѧ����NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ����������ʵ�飺| ʵ����� | �������������mL�� | ������������Һ�����mL�� | ||
| ������ | ĩ���� | ������� | ||
| 1 | 15.00 | 0.50 | 17.75 | 17.25 |
| 2 | 15.00 | 0.05 | 16.10 | 16.05 |
| 3 | 15.00 | 0.00 | 15.95 | 15.95 |
���� ��1���ζ���ʹ��ǰӦ�ô�װҺ��ϴ��
��2�����ݵζ��ܵĽṹ�뾫ȷ����������
��3���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��������Һ��ɫ�ı仯�ж��յ㣻
��4������2�ζ���֮�Ϊ����������Һ�����
��5���������ݽϴ��һ�飬Ȼ���������������ƽ��ֵ�������ݹ�ϵʽ��NaOH��HCl�����������������Һ��Ũ�ȣ�
��6������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1���ζ���ʹ��ǰӦ�ô�װҺ��ϴ���Է�ֹ��ϡ�ͣ��ʴ�Ϊ����װҺ��
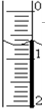
��2���ζ���Һ��Ķ���0.70mL���ʴ�Ϊ��0.70��
��3��ȡ15.00mL��������װ����ƿ�У��μ�2�η�̪��ָʾ�����ζ��DZߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��������Һ��ɫ�ı仯�ж��յ㣻
��ѡb��
��4��������0.50mL��ĩ����17.75mL������������������Һ���Ϊ17.25mL��
�ʴ�Ϊ��17.25��
��5�����εζ����ı�Һ����ֱ�Ϊ��17.251mL��16.05mL��15.95mL����һ���������ϴ�Ӧ����������������ʵ�������ı�Һƽ�����Ϊ��$\frac{16.05mL+15.95mL}{2}$=16.00mL��
NaOH��HCl
0.1020mol/L��16.00mL C��HCl����15.00mL
��ã�C��HCl��=0.1088mol/L
�ʴ�Ϊ��16.00��0.1088��
��6��a����ƿ�м��������Һ���ټ���������ˮ����������Һ�����ʵ������䣬��Һ��������䣬������䣬��a����
b����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��������Һ�����ʵ���ƫС�����V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫС����b����
c������ָʾ������ɫ�б仯��ֹͣ�ζ������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫС����c����
��ѡa��
���� ������Ҫ�����к͵ζ�����Ŀ�ѶȲ�����ȷ�ζ����������ݴ������������ǽ����Ĺؼ���ע����������к͵�ʵ�ʣ�����������ѧ���Ļ�ѧʵ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��һ�ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
����˵����ȷ����
A��H2SO4��H3PO4��Ħ���������
B��2g�������1 mol��
C��O2��Ħ����������ֵ�ϵ�����һ�����ӵ�����
D��1 mol CO��������28 g��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.01 mol•L-1HA����Һ��c��H+��=1��10-4mol•L-1 | |
| B�� | pH=3��HA��Һ��pH=11��NaOH��Һ�������Ϻ�������Һ��c��Na+����c��A-����c��OH-����c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��HA��Һ��NaA��Һ�������Ϻ�������Һ�����ԣ��� c��OH-��-c��H+����c��HA��-c��A-�� | |
| D�� | pH=3��HA��Һ��pH=11��NaOH��Һ�������1��10��Ϻ�������Һ�� c��OH-��+c��A-���Tc��H+��+c��Na+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͬ�¶��£��������Ȼ�������ֱ������ͬ����Ģ�����ˮ ��0.1 mol/L�����0.1 mol/L�Ȼ�þ��Һ ��0.1 mol/L��������Һ�У�Ag+Ũ�ȣ��٣���=�ڣ��� | |
| B�� | �ں���BaSO4��������Һ�м���Na2SO4���壬c��Ba2+������ | |
| C�� | ��Mg��OH��2����Һ�еμ�FeCl3��Һ��������Ϊ���ɫ��˵���ܽ��Mg��OH��2��Fe��OH��3 | |
| D�� | ��֪I3-?I2+I-����ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ǿ�� | B�� | �������� | ||
| C�� | a1 ��ǿ�ᣬa2 ������ | D�� | a1 �����ᣬa2 ��ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
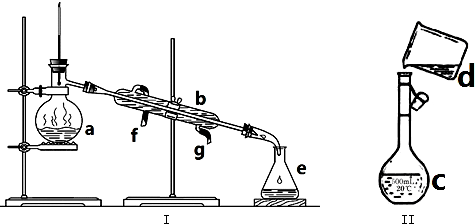
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������������������ֱ���ȫȼ�գ����߷ų��������� | |
| B�� | ��֪C��s��+$\frac{ax}{0.02}$O2��g��=CO��g���ķ�Ӧ��Ϊ110.5kJ/mol��˵��̼��ȼ����Ϊ110.5kJ | |
| C�� | ��֪��ӦX+Y=M+NΪ���ȷ�Ӧ���ʲ��ؼ��ȾͿɷ��� | |
| D�� | ϡ��Һ�У�H+��aq��+OH-��aq��=H2O��l����H=-57.3kJ•mol-1��������0.5 molH2SO4��Ũ��Һ�뺬1 molNaOH����Һ��ϣ��ų�����������57.3KJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com