
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
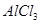 ��Һʱ��ͼ12-5��ʾ�ĵ��仯���ߺ�������
��Һʱ��ͼ12-5��ʾ�ĵ��仯���ߺ�������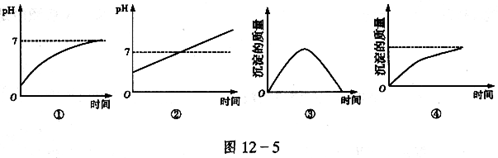
| A���٢� | B���ڢ� | C���ڢ� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Դ�����������ǵ��۵������� | B�����Դ�����������ǵ��۵ĸ����� |
| C�����۵�����������ԭ��Ӧ�� | D�����Ӵӵ�Դ�ĸ����ص���������۵������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��״��������֪��Au��HNO3��4HCl===H[AuCl4]��NO����2H2O��
��״��������֪��Au��HNO3��4HCl===H[AuCl4]��NO����2H2O��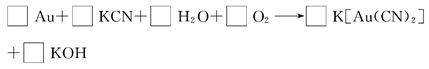
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
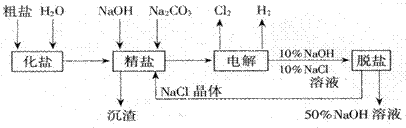
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����̬�Ȼ��� | B������״̬���Ȼ�þ |
| C����ˮ | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2��Cl2 | B��Cu��O2 |
| C��Cu��Cl2 | D��H2��O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ˮ����ΪAl2+ˮ�����ɵ�Al��OH��3�������к�ǿ�������� |
| B���ϳɰ����ɹ����У����ø��¸�ѹ����Ϊ�����N2��H2ת���� |
| C����������ͭʱ����ͭ������ |
| D����ⱥ��ʳ��ˮ���ռNaOH������������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com