
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
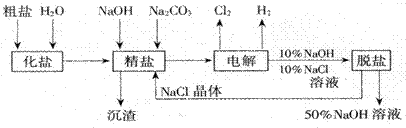
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������100 g������128 g | B������93.6 g������121.6 g |
| C������91.0 g������119.0 g | D������86.0 g������114.0 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | Na+��K+��Cu2+ |
| ������ | SO42����OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
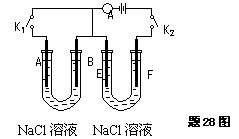
�鿴�𰸺ͽ���>>
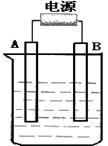
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڵ��ص�����������ԭ��Ӧ |
| B�����Դ�����������ǵ��ص����� |
| C���������Һ�е�������������ص����� |
| D�����Ӵӵ�Դ�ĸ����ص���������ص����� |
�鿴�𰸺ͽ���>>
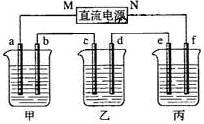
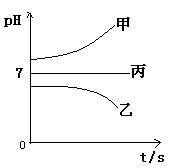
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������У��������õ������壬����ͬ�������������� |
| B���������У�����ͬ�������£��������õ������������������Ĵ� |
| C����������ת�Ƶ��ӵ����ʵ���ԼΪ8mol |
| D��ԭijŨ�ȵ�NaCl��Һ������117g NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ǵ���ʵı�����������ˮ��Һ���ۻ�״̬���ܷ���� |
| B��ǿ�������������ʵı�����������ˮ��Һ�����Եļ��� |
| C���ᡢ���������ڵ���ʣ����������ﶼ�Ƿǵ���� |
| D��������ǿ�ᡢǿ��ʹ��ζ���ǿ����ʣ����������ﶼ�Ƿǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
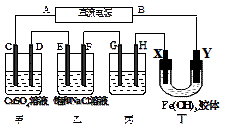
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com