
ijͭ��ʯ��Ҫ��Cu2(OH)2CO3����������Fe��Si�Ļ����ʵ�����Դ�ͭ��ʯΪԭ���Ʊ�CuSO4��5H2O��CaCO3�����ֲ������£�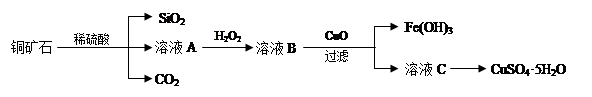
��ش��������⣺
��1����ҺA������Cu2+�⣬�����ܺ��еĽ���������________(�����ӷ���)������ҺA�м���H2O2��Ӧ�����ӷ���ʽ��______________��
��2���������ɵ�CO2��ȡ����̼��ơ��Ʊ�ʱ�������Ȼ�����Һ��ͨ�백������ͨ��CO2��
��ʵ����ͨ�����ü����Ȼ�狀��������ƻ����ķ�����ȡ������ijѧϰС��ѡȡ��ͼ��������װ����ȡ���ռ������İ�����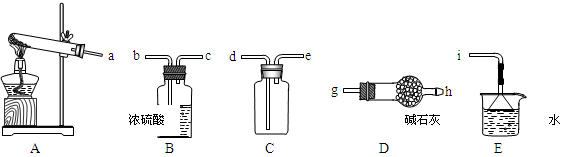
����������������Ӹ������ӿڣ�����Ϊ��ȷ��˳��Ϊa��______��______��______��______�� i��������i����©����������______________��
��ʵ�����л����ù����������ƺ�Ũ��ˮ��ȡ�����������������ʺ���ɸ�ʵ��ļ���װ����
_________(����)
��3���ⶨͭ��ʯ��Cu2(OH)2CO3�����ٷֺ����ķ����ǣ�a����1.25gͭ��ʯ��ȡ��CuSO4��5H2O����ƿ�У���������ˮ��ȫ�ܽ⣻b������Һ�м���100mL0.25mol/L������������ҺʹCu2+��ȫ������c�����ˣ�d����Һ�е�����������Һ��0.5mol/L����ζ����յ㣬����10mL���ᡣ��ͭ��ʯ��Cu2(OH)2CO3��������Ϊ_____________��
��1��Fe2+��Fe3+��2�֣���2Fe2++H2O2 +2H+��2Fe3++2H2O ��2�֣�
��2����a��g��h��e��d�� i ��2�֣�����ֹ������1�֣�����A��2�֣� ��3��88.8%��2�֣�
���������������1��Cu2(OH)2CO3�Լ�Fe��Si�Ļ�������ϡ���ᷴӦ��������ͭ������������������������������ϡ�����Ӧ��������Һ��A�г�����Cu2+�⣬�����ܺ��еĽ���������Fe2+��Fe3+��˫��ˮ����ǿ�����ԣ��ܰ����������������������ӣ���������ҺA�м���H2O2��Ӧ�����ӷ���ʽ��2Fe2++H2O2 +2H+��2Fe3++2H2O��
��2���ٸ���װ��ͼ��֪��Aװ�����Ʊ������ġ��������ɵİ����к���ˮ������������Ҫ���ѡ�ü�ʯ�Ҹ���������ܶ�С�ڿ����ģ��Ұ�����������ˮ������Ӧ���������ſ������ռ�������Ҫ������İ����������գ������ȷ�IJ���˳����a��g��h��e��d�� i��������������ˮ�������i������©���������Ƿ�ֹ������
���ù����������ƺ�Ũ��ˮ��ȡ���������������Ҫ��Һ©������Ӧ����Ҫ���ȣ��Ұ����������ſ������ռ���������ȷ�Ĵ�ѡA��
��3��������������ʵ�����0.01L��0.5mol/L��0.005mol������ݷ���ʽNaOH��HCl��NaCl��H2O��֪�������ᷴӦ������������0.005mol���������Ƶ����ʵ�����0.1L��0.25mol/L��0.025mol����������ͭ��Ӧ������������0.025mol��0.005mol��0.020mol������ݷ���ʽ2NaOH��CuSO4��Cu(OH)2����Na2SO4��֪������ͭ�����ʵ�����0.020mol��2��0.010mol�����Ը���ԭ���غ��֪��ͭ��ʯ��Cu2(OH)2CO3�����ʵ�����0.010mol��2��0.005mol������Cu2(OH)2CO3��������Ϊ ��100%��88.8%��
��100%��88.8%��
���㣺����������ԭ����ʽ��ƽ�������Ʊ������ʺ����IJⶨ�Լ�ʵ�鷽������������۵�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(4��)�����й�ʵ��������жϲ���ȷ���� _____________������ţ���ѡ�۷֣���
| A������һ�����ʵ���Ũ����Һ������ʱ���ӿ̶��ᵼ��������ҺŨ��ƫ�� |
| B������Ũ����մ��Ƥ���ϣ�����������������Һ��ϴ |
| C������ϡ����ʱ���������ձ��м���һ�����������ˮ���ٱ�������Ũ����߽��� |
| D��100 mL����ƿ����������95 mL 0.1 mol/L NaCl��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ��������ͭ����ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�衿
��1����_______�����������ƣ���ͬ��ȷ������������������
��2���ڴ������м���Լ2 g��ϸ������ͭ���壬��������
��3����ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������____________����ȴ�����£���������
��4���ظ�(3)��ʵ����к��ز�����ֱ�����γ������������0.001 g��
�����ݼ�¼�봦����
| | ��һ��ʵ�� | �ڶ���ʵ�� |
| ������������g�� | 29.563 | 30.064 |
| ������������������g�� | 31.676 | 32.051 |
| ���غ�����������ͭ��������g�� | 30.911 | 31.324 |
| x��ֵ | 5.05 | 5.13 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ������±�飨��Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʣ��Ʊ�����Mg(ClO3)2��6H2O���������£�
��֪�����ֻ�������ܽ��(S)���¶�(T)�仯��������ͼ��ʾ��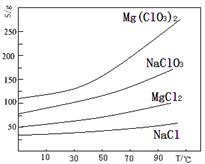
��1������ʱ��Ҫ���������� ������BaCl2��Ŀ���dz�ȥ�������ӣ������ѳ�����ȫ�ķ����ǣ� ��
��3��������pH=4����ѡ�õ��Լ��� (����)
��MgO ��CuO ��ϡNaOH��Һ
��Mg ��ϡ���� ��MgCO3
��4����ӦIIΪ��MgCl2��2NaClO3��Mg(ClO3)2��2NaCl�����ٽ�һ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ���� ���ڳ��ȹ��ˣ��� ���ܹ��ˡ�ϴ�ӡ����
��5����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����m g��Ʒ���100 mL��Һ��
����2��ȡ10 mL��Һ����ƿ�У�����10 mLϡ�����20 mL 1.000 mol��L��1��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol��L��1 K2Cr2O7����Һ�ζ�δ��������Fe2������¼����K2Cr2O7��Һ�������
�ٲ���2�з�����Ӧ�����ӷ���ʽΪ�� ��
��������K2Cr2O7����Һʱδϴ���ձ������Ʒ��Mg(ClO3)2��6H2O�ĺ����� ��(�ƫ�ߡ�����ƫ�͡� ���䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������岻��ȱ�ٵ���Ԫ�أ����뺬���Ļ�����ɲ��������������ơ����г���һ�ֳ����IJ���ҩƷ���±���˵����IJ������ݡ�
[���]ÿƬ������������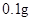 [��Ӧ֢]����ȱ����ƶѪ֢��Ԥ���������á� [�����÷�]����Ԥ���� 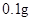 /�գ����������� /�գ�����������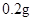 �� ��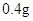 /�ա� /�ա�С������Ԥ���� 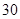 �� ��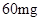 /�գ������� /�գ�������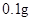 �� ��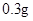 /�� /��[����]�ܹ⡢�ܷ⡢�ڸ��ﴦ���档 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С��Ϊ�о��������ȡ�Ͳ�������ʣ���������ʵ�顣
ʵ��I:�Ʊ�����
ʵ������������������ˮ��Һ�Ʊ������װ����ͼ��ʾ(���ȡ�����������̶�װ�þ�����ȥ����ʵ��������£�
�ٽ�һ�����ĵ���ˮ��Һ��������ƿ��
�ڿ��Ʒ�ӦҺ�¶���55?600C�����£��߽�������μ�һ�����������������Ļ��� (65%HNO3��98%H2S04��������Ϊ2 :1.5)��Һ
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�塣
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O
C6H12O6��8HNO3��6CO2����8NO����10H2O
3 H2C2O4��2HNO3��6CO2����2NO����4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ________��
(2)ʵ����������μӹ��죬�����²�������½�����ԭ����________��
ʵ��II�����ᾧ���нᾧˮ�ⶨ
���ᾧ��Ļ�ѧʽ�ɱ�ʾΪH2C2O4? xH2O,Ϊ�ⶨx��ֵ,��������ʵ�飺
�ٳ�ȡ6.3gij���ᾧ�����100.0mL��ˮ��Һ��
��ȡ25.00mL������Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊ0. 5mol/L��KMnO4��Һ�ζ����ζ��յ�ʱ����KMnO4�����Ϊ10.00mL���ش�����������
��3��д��������Ӧ�����ӷ���ʽ________________��
��4������x=________��
��5���ζ�ʱ�������ַ�Ӧ���ʿ�ʼ�����������ӿ죬���ܵ�ԭ����________��
ʵ��III:����ȶ���
�������ϣ����ᾧ��(H2C2O4 ?xH20),1000C��ʼʧˮ��100.5�����ҷֽ����H2O��CO��CO2��������ͼ���ṩ���������Լ������һ��ʵ�飬֤�����ᾧ��ֽ�õ��Ļ��������H2O��CO��CO2 (����װ�ú͵��ܵ���ͼ����ȥ������װ�ÿ��ظ�ʹ�ã���
�ش��������⣺
(6)����װ�ð�����˳��Ϊ________��
(7)����B����ˮ����ͭ������________��
��8����֤��������к���CO��ʵ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʯ����Ҫ�ɷ�ΪCaCO3��MgCO3������������Fe��Si�Ļ����ʵ��������ʯΪԭ����Mg(OH)2��CaCO3���������£�
ʵ���������Ҫ�����ݼ��±���
| | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Fe2+ | 7.0 | 9.0 |
| Mg2+ | 9.5 | 11.0 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������Ҫ��ҵ���ϡ���ҵ��ͨ���Է���м�����ʲ����ᷴӦ��Ϊԭ���Ʊ�FeCO3���ٽ���������ȡ����������ҵ�Ʊ�FeCO3���������£�
�ش��������⣺
��1��������������� ��
��2��д������FeCO3���������ӷ���ʽ ��
��3����Щͬѧ��Ϊ��Һ������Ԫ�غ�������KMnO4��Һ���ⶨ��5Fe2++MnO-4+8H+=5Fe3++Mn2++4H2O����
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
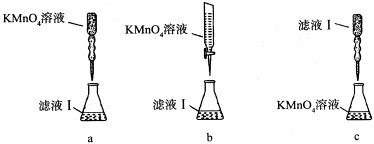
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�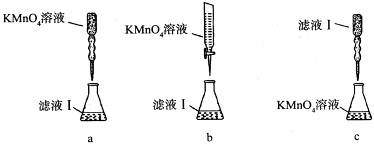
��4�����������õ���Na2CO3�ڹ�ҵ�����г���������NaCl��ijУ��ѧ����������ͼ��ʾװ�����ⶨNa2CO3�ĺ�����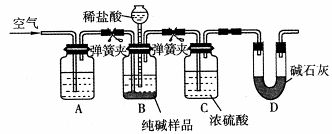
��Ҫ����Na2CO3�����ʵĴ��ڣ�ѡ�������Լ��е� ��ѡ����ţ���
a������������Һ b��ϡ���� c�����������Һ d����������Һ
�ڼ���װ��B�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ���ã��� ��˵��װ�ò�©����
��װ��A�е��Լ� ��װ��C������ ��
������ʵ��װ�ô�������ȱ�ݣ���ȱ�ݵ��²ⶨ���ƫ�ߣ���ȱ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з����п����������к�ˮ��������
| A�����˷� | B����ȡ�� | C����Һ�� | D������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com