
| ʵ����� | ʱ�䣨min�� | ������ƽ�Ķ�����g�� |
| �ձ�+NaOH��Һ | 120.0 | |
| �ձ�+NaOH��Һ+��Ʒ | 135.6 | |
| 1 | 135.1 | |
| 2 | 134.7 | |
| 3 | 134.4 | |
| 4 | 134.4 |

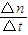 ��������
��������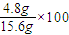 %=30.8%��
%=30.8%��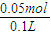 =0.5mol/L���ʴ�Ϊ����Ӧ����Һ�е�c��OH-��Ϊ0.5mol/L��
=0.5mol/L���ʴ�Ϊ����Ӧ����Һ�е�c��OH-��Ϊ0.5mol/L��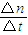 =
=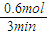 =0.2mol/min��
=0.2mol/min��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������������������Һ��
������������������Һ��| �� | �� | �� | �� | |
| ��Һ | pH=4��NH4Cl | pH=4������ | 0.1mol?L-1�Ĵ��� | 0.1mol?L-1��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��
��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��| ʵ����� | ʱ�䣨min�� | ������ƽ�Ķ�����g�� |
| �ձ�+NaOH��Һ | 120.0 | |
| �ձ�+NaOH��Һ+��Ʒ | 0 | 135.6 |
| 1 | 135.1 | |
| 2 | 134.7 | |
| 3 | 134.4 | |
| 4 | 134.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��
��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��| ʵ����� | ʱ�䣨min�� | ������ƽ�Ķ�����g�� |
| �ձ�+NaOH��Һ | 120.0 | |
| �ձ�+NaOH��Һ+��Ʒ | 0 | 135.6 |
| 1 | 135.1 | |
| 2 | 134.7 | |
| 3 | 134.4 | |
| 4 | 134.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ʵ����� | ʱ�䣨min�� | ������ƽ�Ķ�����g�� |
| �ձ�+NaOH��Һ | 120.0 | |
| �ձ�+NaOH��Һ+��Ʒ | 0 | 135.6 |
| 1 | 135.1 | |
| 2 | 134.7 | |
| 3 | 134.4 | |
| 4 | 134.4 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com