
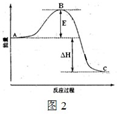
(11��)2SO2(g)��O2(g)  2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 molSO3(g)�Ħ�H����99 kJ/mol��
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 molSO3(g)�Ħ�H����99 kJ/mol��
��ش��������⣺
��1��ͼ��A��C�ֱ��ʾ_____ ___��____ ____��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿________.�÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ�________��������_____________________________��
(2) ͼ�Ц�H��_____ ___kJ/mol.
(3) V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ��_____________________ __
_______________________________________________��
��4�������Ӧ����v(SO2)Ϊ0.05 mol/(L��min)����v(O2)��__________mol/(L��min)��v(SO3)��________ mol/(L��min)��
(5) ��֪�������ȼ����Ϊ296 kJ/mol��������S(s)����3 mol SO3(g)�Ħ�H= ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
 ��2��¯�����Ƶ������ǽ���SO2���г�����ˮϴ��������¯����SO2�Ĵ��������ڽӴ����н��У�
��2��¯�����Ƶ������ǽ���SO2���г�����ˮϴ��������¯����SO2�Ĵ��������ڽӴ����н��У�
| ||
| �� |

| c2(SO3) |
| c2(SO2)c(O2) |
| c2(SO3) |
| c2(SO2)c(O2) |
| ʵ����� | ���������g�� | Ũ�������ӵ�������g�� |
| ��һ�� | 1.570 | 0.340 |
| �ڶ��� | 3.140 | 0.680 |
| ������ | 4.710 | 0.510 |
| ���Ĵ� | 6.280 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ɹŰ������и߶�9���¿���ѧ�Ծ��������棩 ���ͣ������
(11��)2SO2(g)��O2(g)
 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 molSO3(g)�Ħ�H����99 kJ/mol��
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 molSO3(g)�Ħ�H����99 kJ/mol��

��ش��������⣺
��1�� ͼ��A��C�ֱ��ʾ_____ ___��____ ____��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿________.�÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ�________��������_____________________________��
(2) ͼ�Ц�H��_____ ___kJ/mol.
(3) V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ��_____________________ __
_______________________________________________��
��4�������Ӧ����v(SO2)Ϊ0.05 mol/(L��min)����v(O2)��__________mol/(L��min)��v(SO3)��________ mol/(L��min)��
(5) ��֪�������ȼ����Ϊ296 kJ/mol��������S(s)����3 mol SO3(g)�Ħ�H= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ɽ��ģ�� ���ͣ������

| ||
| �� |
| ʵ����� | ���������g�� | Ũ�������ӵ�������g�� |
| ��һ�� | 1.570 | 0.340 |
| �ڶ��� | 3.140 | 0.680 |
| ������ | 4.710 | 0.510 |
| ���Ĵ� | 6.280 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ�����н�ɽ�и߿���ѧģ���Ծ��������棩 ���ͣ������
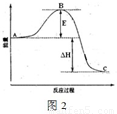
 2SO3��g������Ӧ���̵������仯��ͼ2��ʾ��
2SO3��g������Ӧ���̵������仯��ͼ2��ʾ��| ʵ����� | ���������g�� | Ũ�������ӵ�������g�� |
| ��һ�� | 1.570 | 0.340 |
| �ڶ��� | 3.140 | 0.680 |
| ������ | 4.710 | 0.510 |
| ���Ĵ� | 6.280 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com