��2009?��Ǩ��ģ��ij�о���ѧϰС��Թ���̿������������Ӧ���������ijɷֽ������о���
[�������]�÷�Ӧ�е�������������CO������CO
2��CO�Ļ���
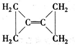
[��������]��������̼��������������Ӧ��ʵ���ҿ������Ȼ�隣�����Һ���������ƣ�NaNO
2��������Һ��ϼ��ȷ�Ӧ�Ƶõ�����
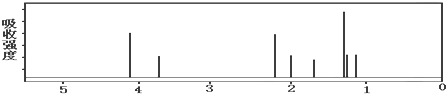
[��Ʒ���]��ͼ��ʾ����һ�������������ڸ��������������������̿����ȫ��Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȣ�

�Իش��������⣺
��l������ͼ����װ�ã���μ���װ�õ�������
������װ��ĩ�˽�һ���䲣�����ܵ��齺�ܣ�������������ĩ�˷���ʢ��ˮ��ˮ���У��þƾ��������巢��װ�������ȣ����ɼУ�����������ĩ�������ݲ�����ֹͣ���Ⱥ�������������һ��ˮ������©��
������װ��ĩ�˽�һ���䲣�����ܵ��齺�ܣ�������������ĩ�˷���ʢ��ˮ��ˮ���У��þƾ��������巢��װ�������ȣ����ɼУ�����������ĩ�������ݲ�����ֹͣ���Ⱥ�������������һ��ˮ������©��
��2�����ƿ��ʢ�ŵ��Լ�Ϊ
Ũ����
Ũ����
��������Ϊ
���ղ����ĵ����е�ˮ����ø���ĵ���
���ղ����ĵ����е�ˮ����ø���ĵ���
��3��ʵ�鿪ʼʱ��Ӧ�ȴ��ɼУ�һ��ʱ���رգ��ٵ�ȼ�ƾ���ƣ�������
Ϊ���ž�����װ���еĿ���
Ϊ���ž�����װ���еĿ���
��
��4����ȡ3.20g��������2.00g̿�ۻ�Ͼ��ȣ���������Ϊ48.48g��Ӳ�ʲ������У�����Ӧ��������ͨһ��ʱ��ĵ�������ȴ�����£��Ƶ�Ӳ�ʲ����ܺ���������Ϊ52.24g������һ���ⶨ��֪�μӷ�Ӧ����Ԫ������Ϊ0.96g���Ӷ�ȷ�ϸ÷�Ӧ�����������C0
2��CO�Ļ���������
�����㷴Ӧ���������̼����ԭ�ӵ����ʵ���֮��Ϊ 2��3������ 1/2 �� 1 ֮��
�����㷴Ӧ���������̼����ԭ�ӵ����ʵ���֮��Ϊ 2��3������ 1/2 �� 1 ֮��
���������ݴ�������жϣ���Ӧ������������n��CO
2����n��CO��=
1��1
1��1
��
��5����ͬѧ����ʵ��ó��Ľ��ۣ���ΪӦ��ʵ��װ�ý�һ�����ƣ�����ΪӦ����θĽ���
��β�����ڴ���һ��ȼ�ľƾ��ƻ�����һβ������װ��
��β�����ڴ���һ��ȼ�ľƾ��ƻ�����һβ������װ��
��




 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�