
��16��)��1��AgNO3��ˮ��Һ�� ����ᡱ�����С���������ԣ�����ʱ��pH 7���>������=������<������ԭ���ǣ������ӷ���ʽ��ʾ����
��
ʵ����������AgNO3����Һʱ������AgNO3���������ڽ�Ũ�������У�Ȼ����������ˮϡ�͵������Ũ�ȣ��� ����ٽ����������ơ�����ˮ�⡣
��2���Ȼ���ˮ��Һ�� �� ��ԭ���ǣ������ӷ���ʽ��ʾ����__________________
_____________________ _____________________________ ��
��AlCl3��Һ���ɣ����գ����õ�����Ҫ��������� ��
��3��������������Һʱ��Ϊ�˷�ֹ����ˮ�⣬���Լ��������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)������ˮ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ��ͳ������ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ�⡣�������ѧ֪ʶ�Ļش�
��1��AΪ0.1 mol��L��1��(NH4)2SO4��Һ���ڸ���Һ�и������ӵ�Ũ���ɴ�С˳��Ϊ ��
��2��BΪ0.1 mol��L��1NaHCO3��Һ����NaHCO3��Һ�д��ڵĸ���ƽ����ϵΪ�������ӷ���ʽ��ʾ���� ��ʵ����NaHCO3��Һ��pH > 7�������NaHCO3��Һ�Լ��Ե�ԭ�� ��
��3��CΪFeCl3��Һ��ʵ����������FeCl3��Һʱͨ����Ҫ�����м��� ��Ŀ���� ��
����B��C��Һ��ϣ����������ɫ��������ɫ���壬�÷�Ӧ�����ӷ���ʽΪ ��
��4��DΪ��������AgCl����ı�����Һ���Ȼ�����ˮ�д��ڳ����ܽ�ƽ�⣺AgCl(S)Ag+(aq)+ Cl��(aq) ��25��ʱ���Ȼ�����Ksp= 1.8��10��10 ��25��ʱ�ֽ������Ȼ����ֱ�����100mL����ˮ�Т�100mL 0.2 mol��L��1AgNO3��Һ�Т�100mL 0.1 mol��L��1�Ȼ�����Һ�Т�100mL 0.1 mol��L��1������Һ�С���ֽ�������ͬ�¶���������Ũ���ɴ�С��˳���� ����д��ţ������������ӵ�Ũ��Ϊ mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�˻��а��Ÿ���ѧ����9��˫�ݼ�⻯ѧ�Ծ����������� ���ͣ������
(16��)����A��B��C��D��E������ˮ�����ֻ��������ɵ��������±�����ÿ������ֻ��һ�Ρ�
| ������ | Ag����Na����Fe3����Al3����Ba2�� |
| ������ | OH����Cl����SO32����NO��SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ĵ�ʡ�ɶ��и����ڶ�������Լ�������Ծ����������� ���ͣ�ʵ����
(16��)ijѧϰС����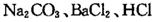 ��Һ���Լ�������ᴿ��������
��Һ���Լ�������ᴿ��������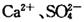 �Ĵ��Ρ���ʵ�鷽�����£�
�Ĵ��Ρ���ʵ�鷽�����£�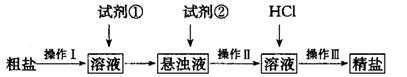
(1) ����I������Ϊ________���Լ���Ӧѡ��_______�������Լ����Ƿ�����ķ���______��
(2) ����I?III���õ�������________�Σ�������Һ�����ε�ʵ������У�ijͬѧδ�������������У����յ��¾��δ���ƫ�͡�������������________________��
(3)�ⶨ��Ʒ������NaCl�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g���ᆱ������ƿ��,����30. OmL����ˮ��
�ڶ�������������ƿ�м��˼���ͻ����ָʾ����
����������Ũ��Ϊ��AgNO3��Һ�����յ�(��Һ��dz��ɫ)������AgNO3��ҺV mL��
��ʢװ���ᆱ�ε��ձ�Ӧ����________�б���(���������ƣ���
���յ�ʱ��Һ��dz��ɫ��ӫ����ָʾ����________��Ӧ(�Ag+����"NO3-��)��
��ijͬѧ�ڵڶ��εζ�ʱ���ζ�ǰ�������Һ��ֱ���ͼ����������V=________mL��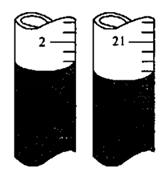
��NaCl����������Ϊ________ (����ĸ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����ڶ��ζο������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
(16��)���ڼס��ҡ�����������������Һ��������Ϣ��
�ٷֱ�NH4+��Na+��Al3+��Ba2+��Ag+��NO3����Cl����SO42-��Br����CO32�������еĸ�һ�����(���ظ�)��
�����мס�������������Һ�����ԣ�����Һ�ʼ��ԡ�
�ۼס��ҷ�Ӧ���ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ������
��ش��������⣺
���û�ѧʽ��ʾ�������ʣ��� ���� ��
���������ӷ���ʽ��ʾ����Һ�����Ե�ԭ�� ��
���������ӷ���ʽ��ʾ�����ҵķ�Ӧ�� ��
�ȼ������Һ�м������ӵķ������ȼ� �Լ����ټ� �Լ����۲쵽
����֤���������Ӵ��ڡ�
�����������γɵİ�ɫ�������ܶȻ�����Ksp=1.8��10��20����1 L 1mol/L�ı���Һ��1 L 1 mol/L�Ķ���Һ��ϳ�ַ�Ӧ��������Һ���ʱ��С����仯���γɳ����������ӵ�Ũ��ԼΪ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ��и����ڶ�������Լ�������Ծ��������棩 ���ͣ�ʵ����
(16��)ijѧϰС����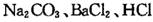 ��Һ���Լ�������ᴿ��������
��Һ���Լ�������ᴿ��������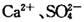 �Ĵ��Ρ���ʵ�鷽�����£�
�Ĵ��Ρ���ʵ�鷽�����£�
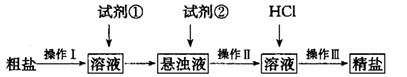
(1) ����I������Ϊ________���Լ���Ӧѡ��_______�������Լ����Ƿ�����ķ���______��
(2) ����I〜III���õ�������________�Σ�������Һ�����ε�ʵ������У�ijͬѧδ�������������У����յ��¾��δ���ƫ�͡�������������________________��
(3)�ⶨ��Ʒ������NaCl�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g���ᆱ������ƿ��,����30. OmL����ˮ��
�ڶ�������������ƿ�м��˼���ͻ����ָʾ����
����������Ũ��Ϊ��AgNO3��Һ�����յ�(��Һ��dz��ɫ)������AgNO3��ҺV mL��
��ʢװ���ᆱ�ε��ձ�Ӧ����________�б���(���������ƣ���
���յ�ʱ��Һ��dz��ɫ��ӫ����ָʾ����________��Ӧ(�Ag+����"NO3-��)��
��ijͬѧ�ڵڶ��εζ�ʱ���ζ�ǰ�������Һ��ֱ���ͼ����������V=________mL��
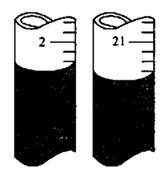
��NaCl����������Ϊ________ (����ĸ��ʾ)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com