
(2)��ͬ���ʵ�����D��G�ֱ����Ʒ�Ӧʱ��������ɫ����������(ͬ��ͬѹ��)Ϊ1��2�������������ʵ����ϵ����ͼ
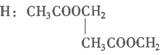
��д����������ʵĽṹ��ʽ��
A.________��B.________��C.________��D.________��E.________��F.________��G.________��H.________��
 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������AC2��B2C2��AD4��Ԫ��A��������ۺ����ϼ۾���ֵ��ȣ�Ԫ��B�ĵ�������C����̬�����о���ȼ�գ�����ʻ�ɫ�������ɵ���ɫ����B2C2��
Ԫ��D�ĸ�һ�������ӵ��Ӳ�ṹ���ԭ����ͬ����
(1)A��B��C��D��Ԫ�ط���ΪA_______________��B_______________��C_______________��D_______________��
(2)AC2��B2C2��AD4�ĵ���ʽ�ֱ�Ϊ_______________��______________��______________��
(3)AC2�ķ��ӿռ乹����_______________������_______________ (����ԡ��Ǽ��ԡ�)���ӡ�AD4���ӵĿռ乹����______________������_____________(����ԡ��Ǽ��ԡ�)���ӡ�
(4)B2C2�мȺ���_______________�����ֺ���_______________��������_______________ (����ӡ����ۡ�)�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���pH=12��ij��Һ�У���ˮ�����c(OH-)Ϊ�� ��
��1.0��10-7 mol��L-1 ��1.0��10-6 mol��L-1 ��1.0��10-2 mol��L-1 ��1.0��10-12 mol��L-1
A���� B���� C���٢� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ���ϴ�ѧ���и�һ��ѧ�����п��Ի�ѧ�� ���ͣ���ѡ��
(4��)��A��B��C��D���ֿ��������ӻ�������ǵ���������Ag+��Na+��Mg2+��Cu2+����������Cl-��OH-��NO3-��SO42-��ÿ������ֻ����һ�Σ�
�ֽ���Һ������ϣ���¼�������£�
A+B����ɫ������B+ D����ɫ������C+D����ɫ����
��(1)A��B��C��D�Ļ�ѧʽ�ֱ���_________��__________��___________��_________��
(2)�ֱ�д�����з�Ӧ�����ӷ���ʽ
A+B��
B+ D��
C+D��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������г�����2010�����һģ�������ۺϣ���ѧ���� ���ͣ�ѡ����
����������ȷ���� �� ��
��3Li+�к��е��������������֮��Ϊ2��1
����C2H6�����м��Թ��ۼ���Ǽ��Թ��ۼ���֮��Ϊ3��1
�۳����£�11.2L�ļ��������к��е��⡢̼ԭ����֮��Ϊ4��1
��5.6g����������������Ӧʧȥ�ĵ�������뷴Ӧ���������ʵ���֮��Ϊ2��1
A���٢� B���ڢ� C���٢� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ĸ�����ѧ�ڵ������¿���ѧ�Ծ� ���ͣ�ѡ����
��֪��101kPaʱ�������ȼ����Ϊ5518kJ��mol��1��ǿ����ǿ����ϡ��Һ�з�����Ӧʱ���к���Ϊ57��3kJ��mol��1���������Ȼ�ѧ����ʽ��д��ȷ���� �� ��
�� C8H18��l��+ O2��g��= 8CO2��g��+9H2O��g������H = -5518kJ��mol��1
O2��g��= 8CO2��g��+9H2O��g������H = -5518kJ��mol��1
�� C8H18��l��+ O2��g��= 8CO2��g��+9H2O��l������H = -5518kJ��mol��1
O2��g��= 8CO2��g��+9H2O��l������H = -5518kJ��mol��1
�� H+ + OH- = H2O����H = -57��3kJ��mol��1
�� NaOH��aq��+ H2SO4��aq��
H2SO4��aq��  Na2SO4��aq��+H2O��l������H= +57��3kJ��mol��1
Na2SO4��aq��+H2O��l������H= +57��3kJ��mol��1
A���٢� B���� C���ڢ� D���ڢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com