
SnCl4�ڴ���Diels��aider��Ӧ���Ӽ�������ֱ������ҪӦ�ã�ij��ѧ�о�С����̽��SnCl4���Ʊ���
[��������]
SnCl4�ڳ���������ɫҺ�壬�е�Ϊ114�棬����ʪ�����㷢��ˮ�ⷴӦ��Sn���۵�Ϊ231�森
[ʵ�鲽��]
��һ��������Sn���Ʊ���������ʯSnO2Ϊԭ�ϣ��ù����Ľ�̿����ԭ�����ڸ����¿��Ƶô�����
�ڶ�����SnCl4���Ʊ������ø��������C12�����ڵ�Sn��Ӧ�Ʊ�SnCl4ͬʱ�ų��������ȣ�
��ͼ��ʵ�����Ʊ�SnCl4��ʵ��װ�ã�

[����˼��]
(1)��һ����Ӧ�Ļ�ѧ����ʽΪ________��
(2)װ��B��C�е�ҩƷ�ֱ�Ϊ________��
(3)����Ӧ����SnCl4ʱ��ӦϨ��________���ľƾ��ƣ�������________��
[ʵ��Ľ�]
��ʦ˵װ��E��Ʋ�����������������������˵���ĵ�Ŀ�ģ�________��
 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����ѣ�CH3OCH3����һ�ָ�Ч����࣬���������������ܵ�����ȼ�ϣ���ҵ���Ʊ�������ʱ�ڴ���Ӧ���У�ѹǿ2.0��10.0MPa���¶�230��280�棩���еķ�ӦΪ��
�����ѣ�CH3OCH3����һ�ָ�Ч����࣬���������������ܵ�����ȼ�ϣ���ҵ���Ʊ�������ʱ�ڴ���Ӧ���У�ѹǿ2.0��10.0MPa���¶�230��280�棩���еķ�ӦΪ��| 0.3��mol/L��0��3mol/L |
| 0.4mol/L��0.2mol/L |
| 9 |
| 8 |
| 0.12mol/L��0.12mol/L |
| 0.08mol/L��0.18mol/L |
| 0.3��mol/L��0��3mol/L |
| 0.4mol/L��0.2mol/L |
| 9 |
| 8 |
| 0.12mol/L��0.12mol/L |
| 0.08mol/L��0.18mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
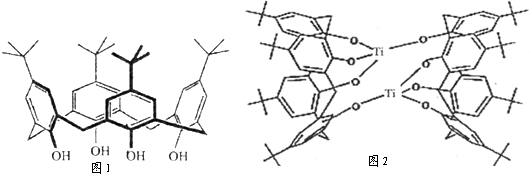
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼΪij�л���Ľṹ��ʽ����֪������ͭ�������CuO?CuCrO4�����£���������Ӧ�õ������ʻ�˫����ͬʱ����ԭ���������ڴ��⻯�����в��䣬�䷴Ӧԭ�����£�RCOOR��+2H2
��ͼΪij�л���Ľṹ��ʽ����֪������ͭ�������CuO?CuCrO4�����£���������Ӧ�õ������ʻ�˫����ͬʱ����ԭ���������ڴ��⻯�����в��䣬�䷴Ӧԭ�����£�RCOOR��+2H2| ͭ�������� |
| A�����л���Ļ�ѧʽΪC20H14O5 |
| B��1mol���л�����Ũ��ˮ��Ӧ������5molBr2 |
| C������������������Һ��ַ�Ӧ������4mol�������� |
| D��1mol���л�����ͭ���������������3mol����������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0119 �¿��� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com