
����Ŀ����.������ Mg5Al3(OH)19(H2O)4 ������������ȼ���ϣ�����ʱ�����»�ѧ����ʽ�ֽ⣺2Mg5Al3(OH)19(H2O)4![]() 27H2O��+10MgO+3Al2O3
27H2O��+10MgO+3Al2O3
(1)д���û���������ȼ������������___________��
(2)�����ӷ���ʽ��ʾ��ȥ��������� Al2O3 ��ԭ��___________
(3)��֪ MgO������NH4Cl��ˮ��Һ���û�ѧ����ʽ��ʾ��ԭ��_____________��
��.���Բ��� A(M=296g/mol)��������Ԫ����ɵĻ�������ij�о�С�鰴��ͼ����̽ ������ɣ�

��ش�
(4)A�Ļ�ѧʽΪ_____��C �гʻ�ɫ�����Ӷ�ӦԪ����Ԫ�����ڱ��е�λ��Ϊ__________��
(5)��֪������ A ����ϡ���ᷴӦ������һ�ֵ���ɫ�������һ������(����������������������ܶ�Ϊ2)����������ӵĵ���ʽΪ_____��д���÷�Ӧ�����ӷ���ʽ_________��
(6)д��F��G��Ӧ�Ļ�ѧ����ʽ____________��
���𰸡���Ӧ���Ƚ����¶����������������������ˮ����ϡ�Ϳ��� Al2O3 +2OH=2AlO2+H2O NH4Cl+H2O![]() NH3H2O+HCl��MgO+2HCl=MgCl2+H2O ��MgO+H2O+ 2NH4Cl=MgCl2+2NH3H2O Fe3S4 �������ڵ�����
NH3H2O+HCl��MgO+2HCl=MgCl2+H2O ��MgO+H2O+ 2NH4Cl=MgCl2+2NH3H2O Fe3S4 �������ڵ����� ![]() Fe3S4+6H+=3H2S��+3Fe2++S H2SO3+I2+H2O=H2SO4+2HI
Fe3S4+6H+=3H2S��+3Fe2++S H2SO3+I2+H2O=H2SO4+2HI
��������
����(1)�ֽⷴӦ�����ȷ�Ӧ�����ɵ�����þ�����������۵�ߣ�
(2)����þ�Ǽ����������������������������ݶ������ʲ�ͬ��ȥ��
(3)�Ȼ����Һ��笠�����ˮ����Һ�����ԣ�����þ������ٽ�ˮ��ƽ��������У�
����C����KSCN��DΪѪ��ɫ��Һ����֪CΪFeCl3��DΪFe(SCN)3�ȣ����ƿ�֪BΪFe2O3����n(Fe2O3)=![]() =0.03mol��n(Fe)=0.06mol��m(Fe)=0.06mol��56g/mol=3.36g��A�������ɵ���ɫ����E��Һˮ�õ�������Һ��������KI��Һ���õ���ɫ��ҺG��������Һ��������ǿ��˵���������E��ˮ��Һ��EӦΪSO2��FΪH2SO3��G���к�H2SO4��HI����֪A����Fe��SԪ�أ���m(S)=5.920g-3.36g=2.56g��n(S)=
=0.03mol��n(Fe)=0.06mol��m(Fe)=0.06mol��56g/mol=3.36g��A�������ɵ���ɫ����E��Һˮ�õ�������Һ��������KI��Һ���õ���ɫ��ҺG��������Һ��������ǿ��˵���������E��ˮ��Һ��EӦΪSO2��FΪH2SO3��G���к�H2SO4��HI����֪A����Fe��SԪ�أ���m(S)=5.920g-3.36g=2.56g��n(S)=![]() =0.08mol����֪n(Fe)��n(S)=3��4�����Դ˽�������
=0.08mol����֪n(Fe)��n(S)=3��4�����Դ˽�������
I.(1)2Mg5Al3(OH)19(H2O)4![]() 27H2O��+10MgO+3Al2O3���ֽⷴӦ�����ȷ�Ӧ���ή���¶ȣ��������ɵ�����þ�������������۵�ܸߵ���������ű������ֹȼ�գ�ˮ����ϡ�Ϳ���������������Ũ�ȣ�����������ȼ����
27H2O��+10MgO+3Al2O3���ֽⷴӦ�����ȷ�Ӧ���ή���¶ȣ��������ɵ�����þ�������������۵�ܸߵ���������ű������ֹȼ�գ�ˮ����ϡ�Ϳ���������������Ũ�ȣ�����������ȼ����
(2)����þ�Ǽ��������������ᣬ�����������������������ᡢ���ڼ�������������ܽ����˳�ȥ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O��
(3)�Ȼ����Һ��笠�����ˮ����Һ�����ԣ�����þ����ˮ�����ɵ��ᣬ��Ӧ�Ļ�ѧ����ʽΪ��NH4Cl+H2O![]() NH3H2O+HCl��MgO+2HCl=MgCl2+H2O��MgO+2NH4Cl+H2O=MgCl2+2NH3H2O��
NH3H2O+HCl��MgO+2HCl=MgCl2+H2O��MgO+2NH4Cl+H2O=MgCl2+2NH3H2O��
II.C����KSCN��DΪѪ��ɫ��Һ����֪CΪFeCl3��DΪFe(SCN)3�ȣ���֪BΪFe2O3����n(Fe2O3)=![]() =0.03mol��n(Fe)=0.06mol��m(Fe)=0.06mol��56g/mol=3.36g��Aȼ�����ɵ���ɫ����E����ˮ�õ�������Һ��������KI��Һ���õ���ɫ��Һ������Һ������ǿ��˵���������E��ˮ��Һ��EӦΪSO2��FΪH2SO3��G���к�H2SO4��HI����֪A����Fe��SԪ�أ���m(S)=5.920g-3.36g=2.56g��n(S)=
=0.03mol��n(Fe)=0.06mol��m(Fe)=0.06mol��56g/mol=3.36g��Aȼ�����ɵ���ɫ����E����ˮ�õ�������Һ��������KI��Һ���õ���ɫ��Һ������Һ������ǿ��˵���������E��ˮ��Һ��EӦΪSO2��FΪH2SO3��G���к�H2SO4��HI����֪A����Fe��SԪ�أ���m(S)=5.920g-3.36g=2.56g��n(S)=![]() =0.08mol����֪n(Fe)��n(S)=3��4������AΪFe3S4��
=0.08mol����֪n(Fe)��n(S)=3��4������AΪFe3S4��
(4)�����Ϸ�����֪��AΪFe3S4�����е���Ԫ��λ��Ԫ�����ڱ��ĵ������� �ڢ�����
(5)������A����ϡ���ᷴӦ������һ�ֵ���ɫ�������һ�����壬����ɫ������ΪS���������Է�������ΪNH3��2������Է���������17��2=34��ΪH2S���壬�����ʽΪ![]() ����Ӧ�����ӷ���ʽΪFe3S4+6H+=3Fe2++S+3H2S����
����Ӧ�����ӷ���ʽΪFe3S4+6H+=3Fe2++S+3H2S����
(6)I2�������ԣ�����Һ�н�H2SO3����Ϊ���ᣬ����F��G��Ӧ�Ļ�ѧ����ʽΪH2SO3+I2+H2O=H2SO4+2HI��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б���������ǣ� ��
A. CS2�ĽṹʽΪ��S��C��S
B. 6CO2��6H2O![]() C6H12O6��6O2 ���ñ仯�й���ֱ��ת��Ϊ��ѧ��
C6H12O6��6O2 ���ñ仯�й���ֱ��ת��Ϊ��ѧ��
C. CO2��g����C��s��![]() 2CO��g����H��0����S��0���÷�Ӧ���������Է�����
2CO��g����H��0����S��0���÷�Ӧ���������Է�����
D. NH3ˮ��Һ�ʼ��Ե�ԭ����NH3��H2O![]() NH3��H2O
NH3��H2O![]() NH4����OH��
NH4����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йص绯ѧװ�õ�������ȷ���� ( )

A. ͼ1�У�Zn��MnO2�ɵ�طŵ�ʱ��MnO2������
B. ͼ2�У���⾫��ͭʱ���������ٵ��������������ӵ�����һ�����
C. ͼ4�У��ڸֲ��ϵ��������������Al��ClԪ��ֻ��AlCl4����Al2Cl7����ʽ���ڣ���������ӦʽΪ��Al ��3e����7AlCl4��=4Al2Cl7��
D. ͼ3�У�K�ֱ���M��N���ӣ����ɱ���Fe�缫������Mʱ��Ϊ������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��������CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��

��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ��ݴ˻ش����⣺
�ٵ�Դ��N��Ϊ�ߣߣߣߣߣ���
�ڵ缫b�Ϸ����ĵ缫��ӦΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����ʽ����缫b�����ɵ������ڱ�״���µ������
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ܵ缫c�������仯�ǣߣߣߣߣߣߣߣߣ�g��
�ݵ��ǰ�����Һ���ᡢ���Դ�С�Ƿ����仯��������ԭ��
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£���һ��2 L���ܱ������У�����4 mol A��2 mol B�������·�Ӧ��3A(g)+2B(g)![]() 4C(s)+2D(g)����Ӧһ��ʱ���ﵽƽ�⣬�������1.6 mol C������˵����ȷ��
4C(s)+2D(g)����Ӧһ��ʱ���ﵽƽ�⣬�������1.6 mol C������˵����ȷ��
A. �÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��![]()
B. ��ʱB��ƽ��ת������40%
C. �������ϵ��ѹǿ��ƽ�������ƶ�����ѧƽ�ⳣ������
D. ����B��ƽ�������ƶ���B��ƽ��ת��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���������ȷ����(����)
A. ���Ǹ������������ȣ���Ҫ�ӵ�ʯ����
B. ���Թ����Һ����ȣ�Һ������һ�㲻�����Թ��ݻ���2/3
C. �Թܺ��ձ�������ֱ���ڻ����ϼ���
D. ���Ⱥ��������Ҫ������ǯ��ȡ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ��F��һ�ָ߾������ʳƷ��װ���IJ��ϣ�G������ˮ�ܱ����ɫ��E�Ǿ���ˮ����ζ�Ļ������һ�������£��л���A��B��C��D��E��F��G��H��������б仯��ϵ��

��1��A��D�����еĹ��������Ʒֱ���________��_________��
��2��H��Һ�м������Ƶ�Cu(OH)2�����ȣ��ɹ۲쵽��������_________��ҽѧ�����ô�ԭ�����������__________��
��3��д����Ӧ�ڡ��۵Ļ�ѧ����ʽ����ָ����Ӧ���ͣ�
��_________________��__________��
��_________________��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С������ͼ��ʾװ����ȡ������������ش��������⣺

��1��Ϊ��ֹ�Թ�a��Һ����ʵ��ʱ�������У�����ǰӦ��ȡ�Ĵ�ʩΪ��_____��
��2��װ���е����θ���ܳ������������⣬������һ��Ҫ������_____��
��3���Թ�b�б���̼������Һ�����ó����ܽ��Ҵ������������������ܽ���⣬����_____��
��4�����Թ�b�ռ���һ���������ֹͣ���ȣ���ȥ�Թ�b���������Թ�b�����ú��Թ�b�����۲쵽��ʵ������_____��
��5��ͼ�Ƿ�������������������Ҵ�������ʵ���������ͼ�����Т٢ڢ۱�ű�ʾ�ʵ��ķ��뷽����
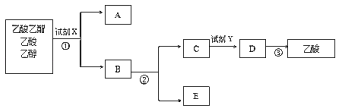
������ʵ������У�����˵����ȷ����_____����ѡ���
A �Լ�XΪ����̼����
B �Լ�Y��Ϊ����
C ��Ϊ��Һ
D �ڢ۾�Ϊ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com