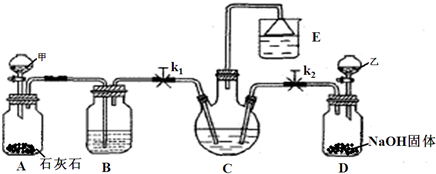
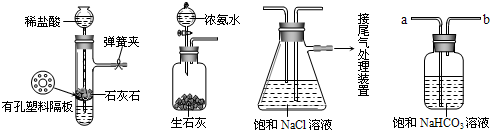
�⣺��1��װ�ü����Ʊ�������̼����ķ�Ӧװ�ã����ɵĶ�����̼�����к����Ȼ������壬���Ʊ�̼��������Ӱ�죬װ���ҵ������������Ȼ������壻����β���к��а��������ŷŵ������У���Ҫ����β�����գ�
�ʴ�Ϊ������HCl��NH
3��
��2����װ�ñ��в�����NaHCO
3�����ķ�ӦΪ��NH
3+CO
2+H
2O+NaCl=NaHCO
3��+NH
4Cl����ȡNa
2CO
3ʱ��Ҫ���˵õ����壬ϴ�Ӻ�������յõ�̼���ƣ�
�ʴ�Ϊ�����ˣ�
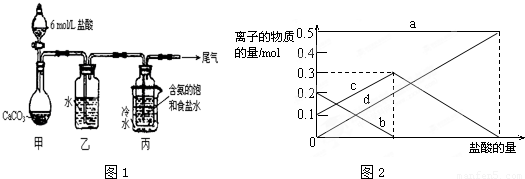
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO
3���ֽⲻ��ȫ����С���һ�ݼ�����t
1min��NaHCO
3��Ʒ����ɽ������о���ȡ������t
1min��NaHCO
3��Ʒ29.6g ��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��裮��������ļ��룬������Ӧ CO
32-+H
+=HCO
3-�� HCO
3-+H
+=CO
2��+H
2O����Һ���й����ӵ����ʵ����ı仯Ϊ̼������Ӽ�С��̼���������Ũ������̼�������ȫ��ת��Ϊ̼��������ӣ��ٵ��������̼��������ӷ�Ӧ���ɶ�����̼��̼��������Ӽ�С������c���߱�ʾ����̼���������Ũ�ȱ仯��̼�������Ũ��0.2mol/L��̼���������Ũ��Ϊ0.1mol/L����Ʒ��NaHCO
3��Na
2CO
3�����ʵ���֮����1��2��
�ʴ�Ϊ��HCO
3-�� 1��2��
��4����ȡ10.5g NaHCO
3�������ʵ���=
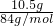
=0.125mol��������t
1min��ʣ����������Ϊ7.4g�����ݻ�ѧ����ʽ���ڵ������仯���㣺
2NaHCO
3=Na
2CO
3+CO
2��+H
2O��m
2 1 62
0.1mol 0.05mol 10.5g-7.4g
��Ӧ��NaHCO
3���ʵ���=0.125mol-0.1mol=0.025mol��NaHCO
3+HCl=NaCl+H
2O+CO
2���������Ȼ������ʵ���0.025mol��
Na
2CO
3���ʵ���=0.05mol��Na
2CO
3+2HCl=2NaCl+H
2O+CO
2���������Ȼ������ʵ���0.1mol��
ʣ���Ȼ������ʵ���=0.200L��1mol/L-0.025mol-0.1mol=0.075mol��ʣ����Һ��c��H
+��=
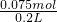
=0.375mol/L
�ʴ�Ϊ��0.375mol/L
��������1��װ�ü����Ʊ�������̼�������к����Ȼ�������Ժ���ʵ��������ţ���Ҫ��ȥ��β���к��а����������ŵ������У���Ҫ���գ�
��2��װ�ñ����ǰ����ı���ʳ��ˮ��ͨ�������̼����̼�����ƾ��壬ͨ�����˵õ�����ϴ�����յõ�̼���ƣ�
��3���������̼���ƺ�̼�����ƣ��������ᷢ����ӦCO
32-+H
+=HCO
3-�� HCO
3-+H
+=CO
2��+H
2O������ͼ�����̼������Ӽ�С��̼������������ࣻ
��4�����ݷ�Ӧǰ�������仯��̼�����Ʒֽ��ԭ�����ݷ�Ӧǰ�������仯���㷴Ӧ��̼�����ƺ����ɵ�̼���ƣ����������������ʣ��̼�����ƣ�����õ�����ﷴӦ���ĵ������ӵõ���
���������⿼���� ��ҵ�ƴ����ԭ�����������������е����ʱ仯�������ɷֵķ����жϺͼ���Ӧ�ã�ʵ����̷��������Ӳ�����β�����գ�ͼ���������жϣ���Ŀ�Ѷ��еȣ�


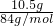 =0.125mol��������t1min��ʣ����������Ϊ7.4g�����ݻ�ѧ����ʽ���ڵ������仯���㣺
=0.125mol��������t1min��ʣ����������Ϊ7.4g�����ݻ�ѧ����ʽ���ڵ������仯���㣺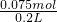 =0.375mol/L
=0.375mol/L

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�