
| ��ѧ�� | Cl��Cl | H��H | H��Cl | N��N |
| ����/kJ��mol | 243 | 436 | 431 | 946 |
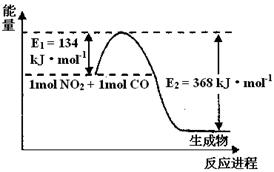
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
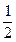 O2��g��====H2O��l����H����285kJ��mol��1
O2��g��====H2O��l����H����285kJ��mol��1 ����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1
����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1 ��====H2O��g�� ��H����44.0kJ��mol��1��д������ȼ������CO2����̬ˮ���Ȼ�ѧ����ʽ_________________________________________��
��====H2O��g�� ��H����44.0kJ��mol��1��д������ȼ������CO2����̬ˮ���Ȼ�ѧ����ʽ_________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)��H2O(l) ����H 3����285.8kJ/mol
O2(g)��H2O(l) ����H 3����285.8kJ/mol | A��488.3 kJ/mol | B����488.3 kJ/mol | C����244.15 kJ/mol | D��244.15 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 aʱ��lmol H2��ȫȼ������Һ̬ˮ���ų�2
aʱ��lmol H2��ȫȼ������Һ̬ˮ���ų�2 85��8kJ��������1 mol CH4��ȫȼ������Һ̬ˮ��CO2���壬�ų�890��3kJ�������������Ȼ�ѧ����ʽ��д��ȷ���� �� ��
85��8kJ��������1 mol CH4��ȫȼ������Һ̬ˮ��CO2���壬�ų�890��3kJ�������������Ȼ�ѧ����ʽ��д��ȷ���� �� ��| A��2H2��g��+ O2��g��=2H2O��1������H=-285��8kJ��mol |
B��CH4��g ��+2 O2��g��=CO2��g��+2H2O��1������H=-890��3kJ��mol ��+2 O2��g��=CO2��g��+2H2O��1������H=-890��3kJ��mol |
| C��CH4��g��+2 O2��g��=CO2��g��+2H2O��g������H=-890��3kJ��mol |
| D��CH4��g��+2O2��g��=CO2��g��+2H2O��1������H=+890��3kJ��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
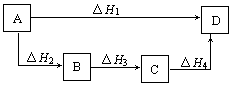
A����H1=��H2=��H3=��H 4 4 | B����H1+��H2=��H3+��H4 |
| C����H1+��H2+��H3=��H4 | D����H1=��H2+��H3+��H4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com