
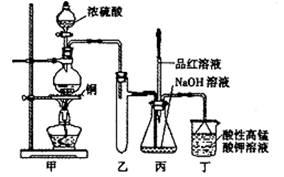
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
| 6.9g |
| 160g/mol |
| 4.1g |
| 18g/mol |
| 0.23 |
| 0.043 |


 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ���� NaHSO3��ʵ��װ����ͼ��ʾ����ش�
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ���� NaHSO3��ʵ��װ����ͼ��ʾ����ش�| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
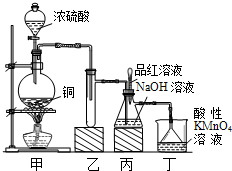 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թ��У��μӹ���lmol/L�Ȼ�����Һ������һ��ʱ��õ���ҺA����B�� | / |
| ����2��������B�м�������ˮϴ�ӳ��������ú���ȥ�ϲ���Һ����������2�Σ���������Ʒ�죬�� |
��Ʒ����ɫ���������ݣ����� |
| ����3�� |
�� �� ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��14�֣�ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ������ͼ��ʾ��
��ش�
��1��ʵ����ȡһ����CuƬ��һ����ŨH2SO4����Բ����ƿ�й��ȣ�����Ӧ����������ƿ�л�������Cuʣ�࣬������Ϊ����һ������H2SO4ʣ�࣬ԭ����
���ڲ�����ŨH2SO4��ǰ���£���ʹʣ��ͭƬ�ܽ���ټ��� ����д�������ڲ�ͬ�������ʣ���
��2����Ӧ�����Һ�м���������CuO��ʹʣ���H2SO4ȫ��ת��ΪCuSO4�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ���壨CuSO4��XH2O��ijС��ͬѧ���ü��ȷ��ⶨ�þ�����ᾧˮX��ֵ��
�������ǵ�ʵ����������ٳ��� �Ρ�
������������һ��ʵ�������
| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
�����ϱ����ݼ����ж�x��ʵ��ֵ������ֵ��x=5�� ���ƫ��ƫС������
��3��װ���ҵ������ǣ� ��
��4������˵����ȷ���ǣ� ������ţ���
a����װ��ʹ�õIJ��������У��ƾ��ơ������ܡ�����©����Բ����ƿ
b��KMnO4��Һ����β������
c������Ʒ����Һ���뵽��ƿ�У���Ʒ�첻��ɫ��˵����NaHSO3����
d������Ʒ����Һ���뵽��ƿ�У���Ʒ����ɫ��˵��NaOH����ȫת��ΪNaHSO3
e������Ʒ����Һ�������Ը��������Һ�������뵽��ƿ�У������Ϻ�ɫ��˵��NaOH����ȫת��ΪNaHSO3
f����װ�û�������ҩƷ������ȡ���ռ���������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com