
| A�� | ��ȡ�ڿ����о��õ�̼���ƾ���143.0g | |
| B�� | ����ʱ�����ӹ۲�̶��� | |
| C�� | ת����Һʱ�������ܽ�̼���ƾ�����ձ�û��ϴ�� | |
| D�� | ���ݺ�����ƿ��ҡ�ȣ�����ʱ����Һ����ڿ̶��ߣ������ּ�����ˮ���̶��� |
���� �������������ʵ����ʵ���n����Һ���V��Ӱ�죬����C=$\frac{n}{V}$���з���������ʹnƫ�����ʹVƫС�IJ������ܹ�ʹ��ҺŨ��ƫ�ߣ�������ҺŨ��ƫ�ͣ��ݴ˽��
��� �⣺A����ȡ�ڿ����о��õ�̼���ƾ���143.0g��Na2CO3•10H2Oʧȥ�ᾧˮ�����³�ȡ�Ĺ����к���̼���Ƶ����ʵ���ƫ����ҺŨ��ƫ�ߣ���Aѡ��
B������ʱ�����ӹ۲�̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���B��ѡ��
C��ת����Һʱ�������ܽ�̼���ƾ�����ձ�û��ϴ�ӣ��������ʲ�����ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���C��ѡ��
D�����ݺ�����ƿ��ҡ�ȣ�����ʱ����Һ����ڿ̶��ߣ������ּ�����ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���D��ѡ��
��ѡ��A��
���� ���⿼����һ�����ʵ���Ũ����Һ�����Ƶ�����������ȷ����ԭ��ȷ���ղ��������ʵ����ʵ���n����Һ���V��Ӱ�죬����C=$\frac{n}{V}$���з������ɽ����Ŀ�ѶȲ���


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
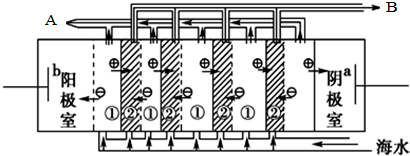 �����������ҿɻ�õ���Ҫ����ԭ�����������������ƣ�
�����������ҿɻ�õ���Ҫ����ԭ�����������������ƣ�| CaSO4 | CaCO3 | BaSO4 | BaCO3 |
| 2.6��10-2 | 7.8��10-4 | 2.4��10-4 | 1.7��10-3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
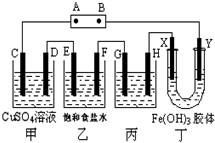 ����ʽ�о���ѧϰ������ѧ������˼ά�����÷�����ij�о���ѧϰС�齫����װ����ͼ���ӣ�C��D��E��F��X��Y ���Ƕ��Ե缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ���Իش��������⣺
����ʽ�о���ѧϰ������ѧ������˼ά�����÷�����ij�о���ѧϰС�齫����װ����ͼ���ӣ�C��D��E��F��X��Y ���Ƕ��Ե缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ���Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ�������c��H+�����ܣ��ۣ��٣��� | |
| B�� | �١��ں͢۵������Ϻ����Һ��pH=7 | |
| C�� | c��NH4+�����ۣ��ܣ��� | |
| D�� | �ٺ͢ڵ������Ϻ����Һ��c��NH4+��+c��NH3•H2O��=0.1mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
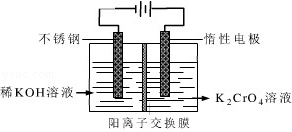 ��������������Ԫ�أ�����֬���л��������ϵ������������������Ⱦ��Σ�����ཡ����
��������������Ԫ�أ�����֬���л��������ϵ������������������Ⱦ��Σ�����ཡ����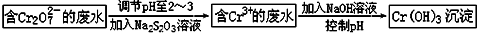
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
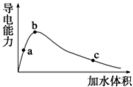 25��ʱ����һ�����ı����ᣨ����ˮ���ᣩ��ˮϡ�ͣ�ϡ��������Һ�ĵ����Ա仯��ͼ��ʾ��������˵��������ǣ�������
25��ʱ����һ�����ı����ᣨ����ˮ���ᣩ��ˮϡ�ͣ�ϡ��������Һ�ĵ����Ա仯��ͼ��ʾ��������˵��������ǣ�������| A�� | ����ĵ���ȣ�a��b��c | |
| B�� | ��Һ��c��H+����b��a��c | |
| C�� | a��b��c�������Һ����c��H+��=c��CH3COO-��+c��OH-�� | |
| D�� | ��b�㵽c�㣬��Һ��$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$�ı�ֵ��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ�������c��H ���T10-13mol/L ��Һ��Mg2+��Cu2+��SO42-��NO3- | |
| B�� | ��ɫ��Һ�У�Na+��NH4+��Cl-��S2- | |
| C�� | ���������Һ��Fe3+��H+��SO42-��C2H5OH | |
| D�� | ������Һ�У�Fe3+��K+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 36gþ�������ĵ�������ȫȼ�չ�ת�Ƶĵ�����Ϊ3NA | |
| B�� | 1mol�������������ᷴӦת�Ƶ�����Ϊ2 NA | |
| C�� | ��״���£�44.8 L NO��22.4 LO2��Ϻ������з�������Ϊ3NA | |
| D�� | 1 molNa2O��Na2O2�����������������������3NA |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com