
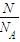 МЦЫуЮяжЪЕФСПЃЌЗжзгЮяжЪЕФСПЮЊ1molЃЌдзгЮяжЪЕФСПЮЊ2molЃЌЫЕУїЪЧЫЋдзгЗжзгЃЛ
МЦЫуЮяжЪЕФСПЃЌЗжзгЮяжЪЕФСПЮЊ1molЃЌдзгЮяжЪЕФСПЮЊ2molЃЌЫЕУїЪЧЫЋдзгЗжзгЃЛ


| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| T/K | T1 | 573 | T2 |
| K | 1.00ЁС107 | 2.45ЁС105 | 1.88ЁС103 |
| 1 |
| 7 |
| ||
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
 ЃЈ2013?КгЮїЧјвЛФЃЃЉыТЃЈN2H4ЃЉКЭАБЪЧЕЊЕФСНжжГЃМћЛЏКЯЮяЃЌдкПЦбЇММЪѕКЭЩњВњжагаЙуЗКгІгУЃЎЧыАДвЊЧѓЛиД№ЯТСаЮЪЬтЃК
ЃЈ2013?КгЮїЧјвЛФЃЃЉыТЃЈN2H4ЃЉКЭАБЪЧЕЊЕФСНжжГЃМћЛЏКЯЮяЃЌдкПЦбЇММЪѕКЭЩњВњжагаЙуЗКгІгУЃЎЧыАДвЊЧѓЛиД№ЯТСаЮЪЬтЃК

| ||
| ||
| T/Ёц | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
| n(NH3) |
| n(CO2) |
| nЃЈN2ЃЉ | nЃЈH2ЃЉ | nЃЈNH3ЃЉ | |
| Мз | 1mol | 3mol | 0mol |
| вв | 0.5mol | 1.5mol | 1mol |
| Бћ | 0mol | 0mol | 4mol |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
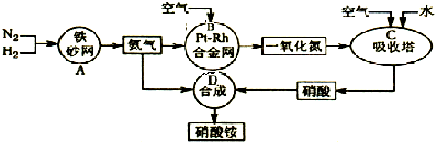
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
34 16 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂHClКЭNaOHЗДгІЕФжаКЭШШЁїH=-57.3kJ/molЃЎдђH2SO4КЭBaЃЈOHЃЉ2ЗДгІЕФжаКЭШШЁїH=2ЁСЃЈ-57.3ЃЉkJ/mol | BЁЂвбжЊC2H5OHЃЈlЃЉ ЕФШМЩеШШЪЧ1366.8KJ/molЃЌдђC2H5OHЃЈlЃЉ+3O2ЃЈgЃЉЈT2CO2ЃЈgЃЉ+3H2OЃЈgЃЉЗДгІЕФЁїH=-1366.8kJ/mol | CЁЂвЛЖЈЬѕМўЯТ2SO2ЃЈgЃЉ+O2ЃЈgЃЉ?2SO3ЃЈgЃЉЁїH1ЃЌ2SO2ЃЈgЃЉ+O2ЃЈgЃЉ?2SO3ЃЈlЃЉЁїH2дђЁїH1ЃОЁїH2 | DЁЂдквЛЖЈЮТЖШКЭбЙЧПЯТЃЌНЋ0.5mol N2КЭ1.5mol H2жУгкУмБеШнЦїжаГфЗжЗДгІЩњГЩNH3ЃЈgЃЉЃЌЗХГіШШСП19.3kJЃЌдђЦфШШЛЏбЇЗНГЬЪНЮЊN2ЃЈgЃЉ+3H2ЃЈgЃЉ?2NH3ЃЈgЃЉЁїH=-38.6kJ/mol |
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com