
 ��ʽ̼����[Cox��OH��y��C03��z]���������Ӳ��ϡ����Բ��ϵ����Ӽ�������ʱ�ɷֽ��������������Ϊ��ȷ������ɣ�ij��ѧ��ȤС��ͬѧ�������ͼ1��ʾ��װ�ý���ʵ�飮
��ʽ̼����[Cox��OH��y��C03��z]���������Ӳ��ϡ����Բ��ϵ����Ӽ�������ʱ�ɷֽ��������������Ϊ��ȷ������ɣ�ij��ѧ��ȤС��ͬѧ�������ͼ1��ʾ��װ�ý���ʵ�飮| ��װ�õ�����/g | ��װ�õ�����/g | |
| ����ǰ | 80.00 | 62.00 |
| ���Ⱥ� | 80.36 | 62.88 |
| 0.36g |
| 18g/mol |
| 0.88g |
| 44g/mol |
| 2.41g |
| 120g/mol |
| 0.36g |
| 18g/mol |
| 0.88g |
| 44g/mol |
| 2.41g |
| 120g/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������Ƶ�Ħ��������40g |
| B��1mol NaOH��������40g/mol |
| C��1g H2��1g N2������������� |
| D����������N2��CH4��CO��H2O��CH4������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
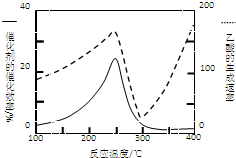 CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ�Ļ�ѧƷ��Ŀǰ���о�����
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ�Ļ�ѧƷ��Ŀǰ���о������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ˮ��Cl2+H2O�T2H++Cl-+ClO- |
| B��������NaHCO3��Һ��Ӧ��H++HCO3-=CO2��+H2O |
| C��������ͭм����Ũ�����еķ�Ӧ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O |
| D������������Һ��ϡ���ᷴӦ��Ba2++SO42-=BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� |
| B������Һ��pH���ڢ���Һ��pH |
| C���ٺ͢ڻ����Һ�У�c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-�� |
| D���ٻ���ж��У�c��HCO3-��+c��CO32-��+c��H2CO3��=0.10 mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ԭ�������ɵ���֮�������� |
| B���������������ɵ���֮��ǿ�ҵ������ |
| C�����ɵ���֮�������� |
| D����������֮�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������Ĵ����ŷ�����ɹ⻯ѧ��������Ҫԭ�� |
| B����鲡��θ�����õı��ͣ��ȿ�����BaSO4��Ҳ������BaCO3 |
| C��Ũ�������������NH3���������ǿ������ |
| D����ˮ��Ʒ����һ��ʱ���pH��4.68��Ϊ4.28������Ϊ�ܽ��˽϶�ĵ��������CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
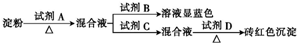
| A��ϡ���ᡢ��ˮ��NaOH��Һ������������ͭ |
| B��ϡ���ᡢ��ˮ��ϡ���ᡢCuSO4��Һ |
| C��NaOH��Һ����ˮ��ϡ���ᡢ����������ͭ |
| D��ϡ���ᡢKI��Һ��NaOH��Һ������������ͭ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com